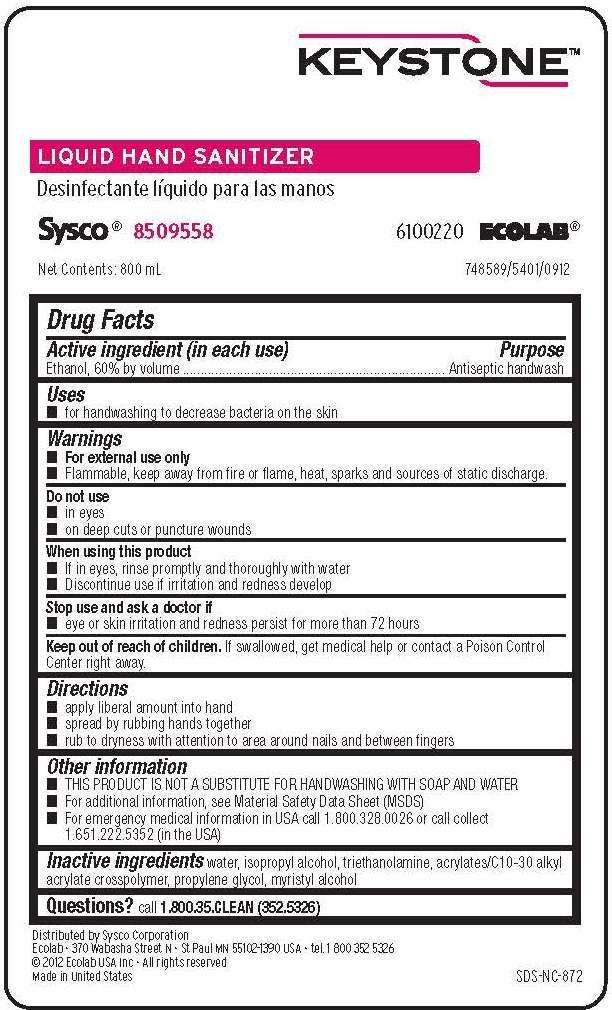
Keystone Liquid Hand Sanitizer
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient (in each use)
- Purpose
- Keystone Liquid Hand Sanitizer Uses
- Warnings
- Directions
- Keystone Liquid Hand Sanitizer Other information
- Principal Display Panel and Representative Label
FULL PRESCRIBING INFORMATION
Active ingredient (in each use)
Ethanol, 60% by volume
Purpose
Antiseptic handwash
Keystone Liquid Hand Sanitizer Uses
- for handwashing to decrease bacteria on the skin
Warnings
- For external use only
- FLAMMABLE. Keep away from fire or flame, heat, sparks and sources of static discharge.
Do not use
- in eyes
- on deep cuts or puncture wounds
When using this product
- If in eyes, rinse promptly and thoroughly with water
- Discontinue use if irritation and redness develop
Stop use and ask a doctor if
- eye or skin irritation and redness persist for more than 72 hours
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- apply liberal amount into hand
- spread by rubbing hands together
- rub to dryness with attention to area around nails and between fingers
Keystone Liquid Hand Sanitizer Other information
- THIS PRODUCT IS NOT A SUBSTITUTE FOR HANDWASHING WITH SOAP AND WATER
- For additional information, see Material Safety Data Sheet (MSDS)
- For emergency medical information in the USA call 1.800.328.0026 or call collect 1.651.222.5352 (in the USA)
Inactive ingredients water, isopropyl alcohol, triethanolamine, acrylates/C10-C30 alkyl acrylate crosspolymer, propylene glycol, myristyl alcohol
Questions? call 1-800-35-CLEAN (352-5326)
Principal Display Panel and Representative Label
Keystone
Liquid Hand Sanitizer
Sysco 8509558
610220 ECOLAB
Net Contents: 800 mL
Distributed by Sysco Corporation - Keystone is a trademark of Ecolab USA Inc.
Ecolab - 370 Wabasha Street N - St. Paul, MN 55102-1390 USA - tel. 1 800 352 5326
2010 Ecolab USA Inc. - All Rights Reserved
Made in United States
Keystone Liquid Hand SanitizerAlcohol SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||