Keystone Antibacterial
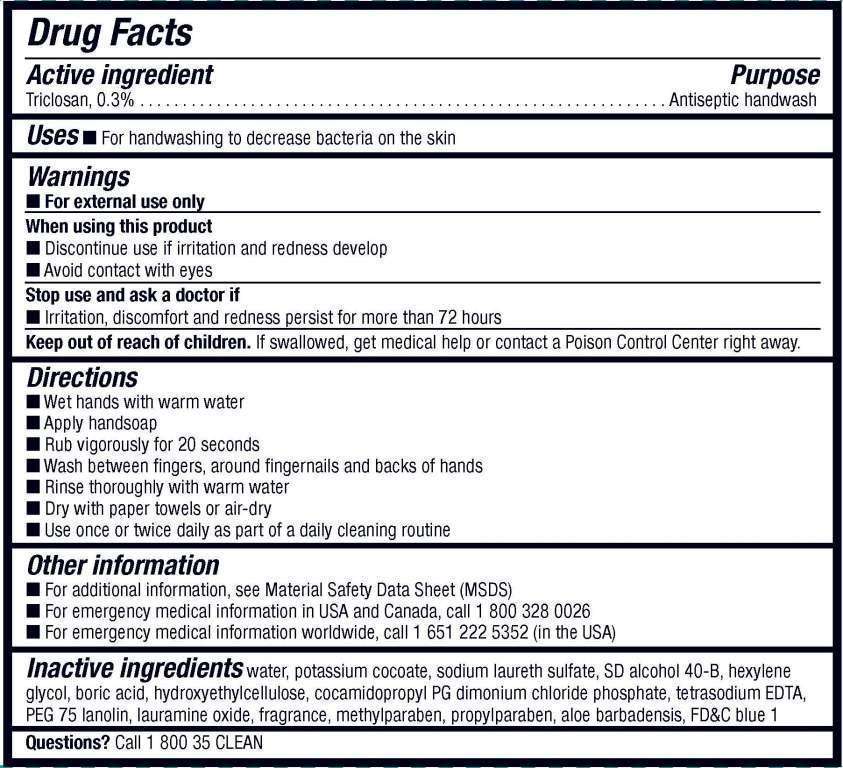
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient
- Purpose
- Keystone Antibacterial Uses
- Warnings
- Directions
- Keystone Antibacterial Other information
- Principal Display Panel and Representative Label
FULL PRESCRIBING INFORMATION
Active ingredient
Triclosan, 0.3%
Purpose
Antiseptic handwash
Keystone Antibacterial Uses
- For handwashing to decrease bacteria on the skin
Warnings
- For external use only
When using this product
- Discontinue use if irritation and redness develop
- Avoid contact with eyes
Stop use and ask a doctor if
- Irritation, discomfort and redness persist for more than 72 hours
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Wet hands with warm water
- Apply handsoap
- Rub vigorously for 20 seconds
- Wash between fingers, around fingernails and backs of hands
- Rinse thoroughly with warm water
- Dry with paper towels or air-dry
- Use once or twice daily as part of a daily cleaning routine
Keystone Antibacterial Other information
- For additional information, see Material Safety Data Sheet (MSDS)
- For emergency medical information in USA and Canada, cal 1-800-328-0026
- For emergency medical information worldwide, call 1-651-222-5352 (in the USA)
Inactive Ingredients water, potassium cocoate, sodium laureth sulfate, SD alcohol 40-B, hexylene glycol, boric acid, hydroxyethylcellulose, cocamidopropyl PG dimonium chloride phosphate, tetrasodium EDTA, PEG 75 lanolin, lauramine oxide, fragrance, methylparaben, propylparaben, aloe barbadensis, FDC blue 1
Questions? Call 1 800 35 CLEAN
Principal Display Panel and Representative Label
KEYSTONE®
KEYSTONE ANTIBACTERIAL LIQUID HAND SOAP
SYSCO™ 7682457
6100079 ECOLAB®
Net Contents 3.78 L (1 US gal)

Keystone AntibacterialTriclosan SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||