Ketorolac Tromethamine
Ketorolac TromethamineInjection, USP
FULL PRESCRIBING INFORMATION: CONTENTS*
- KETOROLAC TROMETHAMINE DESCRIPTION
- CLINICAL PHARMACOLOGY
- CLINICAL STUDIES
- KETOROLAC TROMETHAMINE INDICATIONS AND USAGE
- KETOROLAC TROMETHAMINE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- KETOROLAC TROMETHAMINE ADVERSE REACTIONS
- OVERDOSAGE
- KETOROLAC TROMETHAMINE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- Principal Display Panel
FULL PRESCRIBING INFORMATION
I.V./I.M.
Rx only
WARNING
Ketorolac tromethamine, a nonsteroidal anti-inflammatory drug (NSAID), is indicated for the short-term (up to 5 days) management of moderately severe acute pain that requires analgesia at the opioid level. It is NOT indicated for minor or chronicpainful conditions. Ketorolac tromethamine is a potent NSAID analgesic, and its administration carries many risks. The resulting NSAID-related adverse events can be serious in certain patients for whom ketorolac tromethamine is indicated, especially when the drug is used inappropriately.Increasing the dose of ketorolac tromethamine beyond the label recommendations will not provide better efficacy but will result in increasing the risk of developing serious adverse events.
Gastrointestinal Effects
-
Renal Effects
-
Risk of Bleeding
-
-
Hypersensitivity
-
Intrathecal or Epidural Administration
-
Labor, Delivery and Nursing
-
-
Concomitant Use with NSAIDs
-
DOSAGE AND ADMINISTRATION
Ketorolac Tromethamine Tablets
-
-
Special Populations
-
KETOROLAC TROMETHAMINE DESCRIPTION
Ketorolac tromethamine is a member of the pyrrolo-pyrrole group of nonsteroidal anti-inflammatory drugs (NSAIDs). The chemical name for ketorolac tromethamine is (±)-5-benzoyl-2, 3-dihydro-1H-pyrrolizine-1-carboxylic acid, compound with 2-amino-2-(hydroxymethyl)-1,3-propanediol, and the structural formula is:
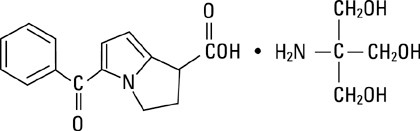
Ketorolac tromethamine is a racemic mixture of [-]S and [+]R ketorolac tromethamine. Ketorolac tromethamine may exist in three crystal forms. All forms are equally soluble in water. Ketorolac tromethamine has a pKa of 3.5 and an n-octanol/water partition coefficient of 0.26. The molecular weight of ketorolac tromethamine is 376.41 and the molecular formula is C19H24N2O6.
Ketorolac tromethamine is available for intravenous (IV) or intramuscular (IM) administration as: 15 mg in 1 mL (1.5%), 30 mg in 1 mL (3%) in sterile solutions; 60 mg in 2 mL (3%) of ketorolac tromethamine in sterile solution is available for IM administration only. The solutions contain 10% (w/v) alcohol, and 6.68 mg, 4.35 mg, and 8.70 mg, respectively, of sodium chloride in sterile water. The pH is adjusted with sodium hydroxide and/or hydrochloric acid, and the solutions are packaged with nitrogen. The sterile solutions are clear and slightly yellow in color.
CLINICAL PHARMACOLOGY
PHARMACODYNAMICS
Ketorolac tromethamine is a nonsteroidal anti-inflammatory drug (NSAID) that exhibits analgesic activity in animal models. Ketorolac tromethamine inhibits synthesis of prostaglandins and may be considered a peripherally-acting analgesic. The biological activity of ketorolac tromethamine is associated with the S-form. Ketorolac tromethamine possesses no sedative or anxiolytic properties.
The peak analgesic effect of ketorolac tromethamine occurs within 2 to 3 hours and is not statistically significantly different over the recommended dosage range of ketorolac tromethamine. The greatest difference between large and small doses of ketorolac tromethamine by either route is in the duration of analgesia.
PHARMACOKINETICS
Ketorolac tromethamine is a racemic mixture of [-]S- and [+]R-enantiomeric forms, with the S-form having analgesic activity.
Comparison of IV, IM and Oral Pharmacokinetics
The pharmacokinetics of ketorolac tromethamine, following IV, IM and oral doses of ketorolac tromethamine, are compared in Table 1. In adults, the extent of bioavailability following administration of the oral and IM forms of ketorolac tromethamine was equal to that following an IV bolus.
Linear Kinetics
In adults, following administration of single oral, IM or IV dosesof ketorolac tromethamine, in the recommended dosage ranges, the clearance of the racemate does not change. This implies that the pharmacokinetics of ketorolac tromethamine in adults, following single or multiple IM, IV, or recommended oral doses of ketorolac tromethamine, are linear. At the higher recommended doses, there is a proportional increase in the concentrations of free and bound racemate.
Distribution
The mean apparent volume (Vβ) of ketorolac tromethamine following complete distribution was approximately 13 liters. This parameter was determined from single-dose data.
The ketorolac tromethamine racemate has been shown to be highly protein-bound (99%). Nevertheless, even plasma concentrations as high as 10 mcg/mL will only occupy approximately 5% of the albumin binding sites. Thus, the unbound fraction for each enantiomer will be constant over the therapeutic range. A decrease in serum albumin, however, will result in increased free drug concentrations.
Ketorolac tromethamine is excreted in human milk (see PRECAUTIONS: Lactation and Nursing).
Metabolism
Ketorolac tromethamine is largely metabolized in the liver. The metabolic products are hydroxylated and conjugated forms of the parent drug. The products of metabolism, and some unchanged drug, are excreted in the urine.
Excretion
The principal route of elimination of ketorolac and its metabolites is renal. About 92% of a given dose is found in the urine, approximately 40% as Metabolites and 60% as unchanged ketorolac. Approximately 6% of a dose is excreted in the feces.
A single-dose study with 10 mg ketorolac tromethamine (n=9) demonstrated that the S-enantiomer is cleared approximately two times faster than the R-enantiomer, and that the clearance was independent of the route of administration. This means that the ratio of S/R plasma concentrations decreases with time after each dose. There is little or no inversion of the R- to S- form in humans. The clearance of the racemate in normal subjects, elderly individuals, and in hepatically and renally impaired patients, is outlined in Table 2.
The half-life of the ketorolac tromethamine S-enantiomer was approximately 2.5 hours (SD±0.4) compared with 5 hours (SD±1.7) for the R-enantiomer. In other studies, the half-life for the racemate has been reported to lie within the range of 5 to 6 hours.
|
% Dose metabolized = <50 % Dose excreted in urine = 91 |
% Dose excreted in feces = 6 % Plasma protein binding = 99 |
1Time-to-peak plasma concentration 2Peak plasma concentration 3Trough plasma concentration 4Average plasma concentration 5Volume of distribution |
|||||||||
|
† Derived from PO pharmacokinetic studies in 77 normal fasted volunteers *Derived from IM pharmacokinetic studies in 54 normal volunteers ‡ Derived from IV pharmacokinetic studies in 24 normal volunteers ††Not applicable because 60 mg is only recommended as a single dose **Mean value was simulated from observed plasma concentration data and standard deviation was simulated from percent coefficient of variation for observed Cmax and Tmax data |
|||||||||||
|
Table 1 Table of Approximate Average Pharmacokinetic Parameters (Mean±SD) Following Oral, Intramuscular and Intravenous Doses of Ketorolac Tromethamine |
|||||||||||
|
Oral † |
Intramuscular* |
Intravenous Bolus ‡ |
|||||||||
|
Pharmacokinetic Parameters (units) |
10 mg |
15 mg |
30 mg |
60 mg |
15 mg |
30 mg |
|||||
|
Bioavailability (extent) |
100% |
||||||||||
|
Tmax 1 (min) |
44±34 |
33±21** |
44±29 |
33±21** |
1.1±0.7** |
2.9±1.8 |
|||||
|
Cmax 2 (mcg/mL) [Single-dose] |
0.87±0.22 |
1.14±0.32** |
2.42±0.68 |
4.55±1.27** |
2.47±0.51** |
4.65±0.96 |
|||||
|
Cmax (mcg/mL) [steady state qid] |
1.05±0.26** |
1.56±0.44** |
3.11±0.87** |
N/A†† |
3.09±1.17** |
6.85±2.61 |
|||||
|
Cmin 3 (mcg/mL) [steady state qid] |
0.29±0.07** |
0.47±0.13** |
0.93±0.26** |
N/A |
0.61±0.21** |
1.04±0.35 |
|||||
|
Cavg 4 (mcg/mL) [steady state qid] |
0.59±0.2** |
0.94±0.29** |
1.88±0.59** |
N/A |
1.09±0.3** |
2.17±0.59 |
|||||
|
Vβ 5 (L/kg) |
—————— 0.175±0.039——————— |
0.210±0.044 |
|||||||||
|
1Estimated from 30 mg single IM doses of ketorolac tromethamine 2Estimated from 10 mg single oral doses of ketorolac tromethamine 3Liters/hour/kilogram |
IV-Administration: In normal subjects (n=37), the total clearance of 30 mg IV-administered ketorolac tromethamine was 0.030 (0.017-0.051) L/h/kg. The terminal half-life was 5.6 (4-7.9) hours. |
|||||||||
|
Table 2 The Influence of Age, Liver and Kidney Function, on the Clearance and Terminal Half-life of Ketorolac Tromethamine (IM 1 and ORAL 2 ) in Adult Populations |
||||||||||
|
Total Clearance [in L/h/kg] 3 |
Terminal Half-life [in hours] |
|||||||||
|
Type of Subjects |
IM Mean (range) |
ORAL Mean (range) |
IM Mean (range) |
ORAL Mean (range) |
||||||
|
Normal Subjects IM (n=54) mean age=32, range=18-60 Oral (n=77) mean age=32, range=20-60 |
0.023 (0.010-0.046) |
0.025 (0.013-0.050) |
5.3 (3.5-9.2) |
5.3 (2.4-9) |
||||||
|
Healthy Elderly Subjects IM (n=13), Oral (n=12) mean age=72, range=65-78 |
0.019 (0.013-0.034) |
0.024 (0.018-0.034) |
7 (4.7-8.6) |
6.1 (4.3-7.6) |
||||||
|
Patients with Hepatic Dysfunction IM and Oral (n=7) mean age=51, range=43-64 |
0.029 (0.013-0.066) |
0.033 (0.019-0.051) |
5.4 (2.2-6.9) |
4.5 (1.6-7.6) |
||||||
|
Patients with Renal Impairment IM (n=25), Oral (n=9) serum creatinine=1.9-5.0 mg/dL, mean age (IM)=54, range=35-71 mean age (Oral)=57, range=39-70 |
0.015 (0.005-0.043) |
0.016 (0.007-0.052) |
10.3 (5.9-19.2) |
10.8 (3.4-18.9) |
||||||
|
Renal Dialysis Patients IM and Oral (n=9) mean age=40, range=27-63 |
0.016 (0.003-0.036) |
− |
13.6 (8-39.1) |
− |
||||||
Accumulation
Ketorolac tromethamine administered as an IV bolus every 6 hours for 5 days to healthy subjects (n=13), showed no significant difference in Cmax on Day 1 and Day 5. Trough levels averaged 0.29 mcg/mL (SD±0.13) on Day 1 and 0.55 mcg/mL (SD±0.23) on Day 6. Steady state was approached after the fourth dose.
Accumulation of ketorolac tromethamine has not been studied in special populations (geriatric, pediatric, renal failure or hepatic disease patients).
Kinetics in Special Populations
Geriatric Patients
Based on single-dose data only, the half-life of the ketorolac tromethamine racemate increased from 5 to 7 hours in the elderly (65 to 78 years) compared with young healthy volunteers (24 to 35 years) (see Table 2). There was little difference in the Cmax for the two groups (elderly, 2.52 mcg/mL±0.77; young, 2.99 mcg/mL±1.03) (see PRECAUTIONS− Geriatric Use).
Pediatric Patients
Following a single intravenous bolus dose of 0.5 mg/kg in 10 children 4 to 8 years old, the half-life was 6 hours (range: 3.5 to 10 h), the average clearance was 0.042 L/hr/kg and the Vd was 0.26 L/kg (range: 0.19 to 0.44 L/kg). In a second study, following a single intravenous dose of 0.6 mg/kg in 24 children 3 to 18 years old, Cmax was 4.3 ± 1.7 mcg/mL, Tmax was 10.25 ± 1.15 minutes, half-life was 3.8 ± 2.6 hours, Cl was 0.0678 L/hr/kg and Vd was 0.25 L/kg. The volume of distribution and clearance of ketorolac in pediatric patients was twice that observed in adult subjects (see Tables 1 and 2). There are no pharmacokinetic data available for ketorolac tromethamine administration by the IM route in pediatric patients.
Renal Insufficiency
Based on single-dose data only, the mean half-life of ketorolac tromethamine in renally impaired patients is between 6 and 19 hours and is dependent on the extent of the impairment. There is poor correlation between creatinine clearance and total ketorolac tromethamine clearance in the elderly and populations with renal impairment (r=0.5).
In patients with renal disease, the AUC∞ of each enantiomer increased by approximately 100% compared with healthy volunteers. The volume of distribution doubles for the S-enantiomer and increases by 1/5th for the R-enantiomer. The increase in volume of distribution of ketorolac tromethamine implies an increase in unbound fraction.
The AUC∞ ratio of the ketorolac tromethamine enantiomers in healthy subjects and patients remained similar, indicating there was no selective excretion of either enantiomer in patients compared to healthy subjects (see WARNINGS – Renal Effects and Table 2).
Hepatic Insufficiency
There was no significant difference in estimates of half-life, AUC∞ and Cmax in 7 patients with liver disease compared to healthy volunteers (see PRECAUTIONS – Hepatic Effects and Table 2).
Race
Pharmacokinetic differences due to race have not been identified.
CLINICAL STUDIES
Adult Patients
The analgesic efficacy of intramuscularly and intravenously administered ketorolac tromethamine was investigated in two postoperative pain models: general surgery (orthopedic, gynecologic and abdominal) and oral surgery (removal of impacted third molars). The studies were double-blind, single- and multiple-dose, parallel trial designs in patients with moderate to severe pain at baseline. Ketorolac tromethamine injection was compared as follows: IM to meperidine or morphine administered intramuscularly, and IV to morphine administered either directly IV or through a PCA (Patient-Controlled Analgesia) pump.
Short-term Use (up to 5 days) Studies
In adults, the comparisons of intramuscular administration during the first hour, the onset of analgesic action was similar for ketorolac tromethamine and the narcotics, but the duration of analgesia was longer with ketorolac tromethamine than with the opioid comparators meperidine or morphine.
In a multi-dose, postoperative (general surgery) double-blind trial of ketorolac tromethamine 30 mg IM versus morphine 6 and 12 mg IM, each drug given on an “as needed” basis for up to 5 days, the overall analgesic effect of ketorolac tromethamine 30 mg IM was between that of morphine 6 and 12 mg. The majority of patients treated with either ketorolac tromethamine or morphine were dosed for up to 3 days; a small percentage of patients received 5 days of dosing.
In clinical settings where perioperative morphine was allowed, ketorolac tromethamine 30 mg IV, given once or twice as needed, provided analgesia comparable to morphine 4 mg IV once or twice as needed.
There was relatively limited experience with 5 consecutive days of IV-administered ketorolac tromethamine in controlled clinical trials, as most patients were given the drug for 3 days or less. The adverse events seen with IV-administered ketorolac tromethamine were similar to those observed with IM-administered ketorolac tromethamine, as would be expected based on the similar pharmacokinetics and bioequivalence (AUC, clearance, plasma half-life) of IV and IM routes of ketorolac tromethamine administration.
Pediatric Patients
The analgesic efficacy of single doses of ketorolac tromethamine has been demonstrated by showing a decrease in the need for supplemental narcotic in pediatric patients receiving ketorolac as compared to placebo. See discussion of these results under Clinical Studies with Concomitant Use of Opioids below.
Clinical Studies with Concomitant Use of Opioids
Adult Patients
Clinical studies in postoperative pain management have demonstrated that ketorolac tromethamine injection, when used in combination with opioids, significantly reduced opioid consumption. This combination may be useful in the subpopulation of patients especially prone to opioid-related complications. Ketorolac tromethamine and narcotics should not be administered in the same syringe.
In a postoperative study, where all patients received morphine by a PCA device, patients treated with ketorolac tromethamine intravenously as fixed intermittent boluses (e.g., 30 mg initial dose followed by 15 mg q3h), required significantly less morphine (26%) than the placebo group. Analgesia was significantly superior, at various postdosing pain assessment times, in the patients receiving ketorolac tromethamine intravenously plus PCA morphine as compared to patients receiving PCA-administered morphine alone.
Pediatric Patients: Ketorolac tromethamine injection reduced the need for supplemental opioid (fentanyl), when a 1 mg/kg dose was administered immediately following tonsillectomy compared to saline controls (see WARNINGS: Hemorrhage). In another study, when a single bolus dose of 0.9 mg/kg of ketorolac tromethamine injection was given to pediatric patients ages 5 to 12 years, compared to saline, a reduction in supplemental opioid was needed following various surgical procedures. In a third study less supplemental morphine was needed in pediatric patients ages 8 to 16 years, who received a 0.8 mg/kg IV injection of ketorolac tromethamine injection in conjunction with morphine following orthopedic surgical procedures, compared to morphine alone. In a study in pediatric patients ages 3 to 12 years, ketorolac tromethamine injection demonstrated a slower onset of analgesia, but a longer duration of action compared to morphine. There is limited data available to support the use of multiple doses of ketorolac tromethamine injection in pediatric patients. There are also insufficient data available to support the use of ketorolac tromethamine tablets in pediatric patients.
Postmarketing Surveillance Study
A large postmarketing observational, non-randomized study, involving approximately 10,000 patients receiving ketorolac tromethamine, demonstrated that the risk of clinically serious gastrointestinal (G.I.) bleeding was dose-dependent (see Table 3A and 3B.) This was particularly true in elderly patients who received an average dose greater than 60 mg/day of ketorolac tromethamine (Table 3A).
|
A. Patients without History of PUB |
||||
|
Age of Patients |
Total Daily Dose of Ketorolac Tromethamine Injection |
|||
|
≤60 mg |
>60 to 90 mg |
>90 to 120 mg |
>120 mg |
|
|
<65 years of age |
0.4% |
0.4% |
0.9% |
4.6% |
|
≥65 years of age |
1.2% |
2.8% |
2.2% |
7.7% |
|
B. Patients with History of PUB |
||||
|
Age of Patients |
Total Daily Dose of Ketorolac Tromethamine Injection |
|||
|
≤60 mg |
>60 to 90 mg |
>90 to 120 mg |
>120 mg |
|
|
<65 years of age |
2.1% |
4.6% |
7.8% |
15.4% |
|
≥65 years of age |
4.7% |
3.7% |
2.8% |
25% |
KETOROLAC TROMETHAMINE INDICATIONS AND USAGE
Adult Patients: Ketorolac tromethamine is indicated for the short-term (≤5 days) management of moderately severe, acute pain that requires analgesia at the opioid level, usually in a postoperative setting. Therapy should always be initiated with IV or IM ketorolac tromethamine, and the oral dosage form is to be used only as continuation treatment, if necessary. Combined use of IV/IM and the oral dosage form is not to exceed 5 days of use because of the potential of increasing the frequency and severity of adverse reactions associated with the recommended doses (see WARNINGS, PRECAUTIONS, DOSAGE AND ADMINISTRATION, and ADVERSE REACTIONS). Patients should be switched to alternative analgesics as soon as possible, but ketorolac tromethamine therapy is not to exceed 5 days.
Pediatric Patients: The safety and effectiveness of single doses of ketorolac tromethamine injection have been established in pediatric patients between the ages of 2 and 16 years. Ketorolac tromethamine as a single injectable dose, has been shown to be effective in the management of moderately severe acute pain that requires analgesia at the opioid level, usually in the postoperative setting. There is limited data available to support the use of multiple doses of ketorolac tromethamine injection in pediatric patients. Safety and effectiveness have not been established in pediatric patients below the age of 2 years. Use of ketorolac tromethamine injection in pediatric patients is supported by evidence from adequate and well-controlled studies of ketorolac tromethamine injection in adults with additional pharmacokinetic, efficacy and safety data on its use in pediatric patients available in the published literature (see CLINICAL PHARMACOLOGY: Clinical Studies, WARNINGS, and PRECAUTIONS).
Ketorolac tromethamine injection has been used concomitantly with morphine and meperidine and has shown an opioid-sparing effect. For breakthrough pain, it is recommended to supplement the lower end of the ketorolac tromethamine injection dosage range with low doses of narcotics prn, unless otherwise contraindicated. Ketorolac tromethamine and narcotics should not be administered in the same syringe (see DOSAGE AND ADMINISTRATION −Pharmaceutical Information for Ketorolac Tromethamine Injection).
KETOROLAC TROMETHAMINE CONTRAINDICATIONS
(see also Boxed WARNING)
-
-
-
-
-
-
-
-
-
-
WARNINGS
(see also Boxed WARNING)
► The combined use of ketorolac tromethamine injection and ketorolac tromethamine tablets is not to exceed 5 days in adults. Only single doses of ketorolac tromethamine injection are recommended for use in pediatric patients.
The most serious risks associated with ketorolac tromethamine are:
-
Gastrointestinal (GI) Effects− Risk of GI Ulceration, Bleeding and Perforation: Ketorolac tromethamine is CONTRAINDICATED in patients with previously documented peptic ulcers and/or G.I. bleeding. Serious gastrointestinal toxicity, such as bleeding, ulceration, and perforation, can occur at any time, with or without warning symptoms, in patients treated with ketorolac tromethamine. Studies to date with NSAIDs have not identified any subset of patients not at risk of developing peptic ulceration and bleeding. Elderly or debilitated patients seem to tolerate ulceration or bleeding less well than other individuals, and most spontaneous reports of fatal G.I. events are in this population. Postmarketing experience with parenterally administered ketorolac tromethamine suggests that there may be a greater risk of gastrointestinal ulcerations, bleeding and perforation in the elderly.
-
The incidence and severity of gastrointestinal complications increases with increasing dose of, and duration of treatment with ketorolac tromethamine. In a non-randomized, in-hospital postmarketing surveillance study, comparing ketorolac tromethamine injection to parenteral opioids, higher rates of clinically serious G.I. bleeding were seen in patients<65 years of age who received an average total daily dose of more than 90 mg of ketorolac tromethamine injection per day (see CLINICAL PHARMACOLOGY − Postmarketing Surveillance Study).
-
The same study showed that elderly (≥65 years of age), and debilitated patients are more susceptible to gastrointestinal complications. A history of peptic ulcer disease was revealed as another risk factor that increases the possibility of developing serious gastrointestinal complications during ketorolac tromethamine therapy (see Tables 3A and 3B).
-
In postmarketing experience, postoperative hematomas and other signs of wound bleeding have been reported in associationwith the perioperative use of ketorolac tromethamine injection. Therefore, perioperative use of ketorolac tromethamine should be avoided and postoperative use be undertaken with caution when hemostasis is critical (see WARNINGS and PRECAUTIONS).
Pediatrics and Tonsillectomy: Physicians should consider the increased risk of bleeding before deciding to administer ketorolac tromethamine injection in patients following tonsillectomy. Ketorolac tromethamine injection is not recommended for use in pediatric patients below the age of 2 years. In a retrospective analysis of patients having undergone tonsillectomy with or without adenoidectomy, the risk of bleeding was 10.1% in patients administered ketorolac tromethamine injection compared to 2.2% in those receiving opioids. The postoperative hemorrhage rate in patients 12 years and younger was 6.5% and 3.3% with and without ketorolac tromethamine injection, respectively. In a prospective study of ketorolac tromethamine injection in pediatric patients (ages 3 to 9 years) undergoing tonsillectomy with or without adenoidectomy, the overall incidence of bleeding was similar between the patients receiving ketorolac tromethamine injection and morphine (16.3% versus 17%, respectively). However, during the first 24 hours after surgery, a higher incidence of bleeding was observed in the ketorolac tromethamine injection group (14.3%) versus the morphine group (4.2%).
-
-
Renal Effects: Ketorolac tromethamine and its metabolites are eliminated primarily by the kidneys, which, in patients with reduced creatinine clearance, will result in diminished clearance of the drug (see CLINICAL PHARMACOLOGY). Therefore, ketorolac tromethamine should be used with caution in patients with impaired renal function (see DOSAGE AND ADMINISTRATION) and such patients should be followed closely. With the use of ketorolac tromethamine, there have been reports of acute renal failure, interstitial nephritis, and nephrotic syndrome.
Because patients with underlying renal insufficiency are at increased risk of developing acute renal failure, the risks and benefits should be assessed prior to giving ketorolac tromethamine to these patients. Hence, in patients with moderately elevated serum creatinine, it is recommended that the daily dose of ketorolac tromethamine injection be reduced by half, not to exceed 60 mg/day. Ketorolac tromethamine is CONTRAINDICATED IN PATIENTS WITH SERUM CREATININE CONCENTRATIONS INDICATING ADVANCED RENAL IMPAIRMENT (see CONTRAINDICATIONS).
Hypovolemia should be corrected before treatment with ketorolac tromethamine is initiated.
-
-
PRECAUTIONS
GENERAL
-
-
INFORMATION FOR PATIENTS
Ketorolac tromethamine is a potent NSAID and may cause serious side effects such as gastrointestinal bleeding or kidney failure, which may result in hospitalization and even fatal outcome.
Physicians, when prescribing ketorolac tromethamine, should inform their patients or their guardians of the potential risks of ketorolac tromethamine treatment (see Boxed WARNING, WARNINGS, PRECAUTIONS, and ADVERSE REACTIONS sections). Advise patients not to give ketorolac tromethamine tablets to other family members and to discard any unused drug.
Remember that the total duration of ketorolac tromethamine therapy is not to exceed 5 (five) days in adults or a single dose in pediatric patients ages 2 to 16 years.
DRUG INTERACTIONS
Ketorolac is highly bound to human plasma protein (mean 99.2%).
Warfarin, Digoxin, Salicylate and Heparin
The in vitro binding of warfarin to plasma proteins is only slightly reduced by ketorolac tromethamine (99.5% control vs 99.3%) when ketorolac plasma concentrations reach 5 to 10 mcg/mL. Ketorolac does not alter digoxin protein binding. In vitro studies indicate that, at therapeutic concentrations of salicylate (300 mcg/mL), the binding of ketorolac was reduced from approximately 99.2% to 97.5%, representing a potential two-fold increase in unbound ketorolac plasma levels. Therapeutic concentrations of digoxin, warfarin, ibuprofen, naproxen, piroxicam, acetaminophen, phenytoin, and tolbutamide did not alter ketorolac tromethamine protein binding.
In a study involving 12 volunteers, ketorolac tromethamine tablets were co-administered with a single dose of 25 mg warfarin , causing no significant changes in pharmacokinetics or pharmacodynamics of warfarin. In another study, ketorolac tromethamine injection was given with two doses of 5000 U of heparin to 11 healthy volunteers, resulting in a mean template bleeding time of 6.4 minutes (3.2 to 11.4 min) compared to a mean of 6.0 minutes (3.4 to 7.5 min) for heparin alone and 5.1 minutes (3.5 to 8.5 min) for placebo. Although these results do not indicate a significant interaction between ketorolac tromethamine and warfarin or heparin, the administration of ketorolac tromethamine to patients taking anticoagulants should be done extremely cautiously, and patients should be closely monitored (see WARNINGS and PRECAUTIONS).
Furosemide
Ketorolac tromethamine administered IV or IM reduced the diuretic response to furosemide in normovolemic healthy subjects by approximately 20% (mean sodium and urinary output decreased 17%).
Probenecid
Concomitant administration of ketorolac tromethamine tablets and probenecid resulted in decreased clearance of ketorolac and significant increases in ketorolac plasma levels (total AUC increased approximately 3-fold from 5.4 to 17.8 mcg/h/mL) and terminal half-life increased approximately 2-fold from 6.6 to 15.1 hours. Therefore, concomitant use of ketorolac tromethamine and probenecid is contraindicated.
Lithium
Inhibition of renal lithium clearance, leading to an increase in plasma lithium concentration, has been reported with some prostaglandin synthesis-inhibiting drugs. The effect of ketorolac tromethamine on plasma lithium has not been studied, but cases of increased lithium plasma levels during ketorolac tromethamine therapy have been reported.
Methotrexate
Concomitant administration of methotrexate and some NSAIDs has been reported to reduce the clearance of methotrexate, enhancing the toxicity of methotrexate. The effect of ketorolac tromethamine on methotrexate clearance has not been studied.
Nondepolarizing Muscle Relaxants
In postmarketing experience, there have been reports of a possible interaction between ketorolac tromethamine injection and nondepolarizing muscle relaxants that resulted in apnea. The concurrent use of ketorolac tromethamine with muscle relaxants has not been formally studied.
ACE Inhibitors
Concomitant use of ACE inhibitors may increase the risk of renal impairment, particularly in volume-depleted patients.
Antiepileptic Drugs
Sporadic cases of seizures have been reported during concomitant use of ketorolac tromethamine and antiepileptic drugs (phenytoin, carbamazepine).
Psychoactive Drugs
Hallucinations have been reported when ketorolac tromethamine was used in patients taking psychoactive drugs (fluoxetine, thiothixene, alprazolam).
Morphine
Ketorolac tromethamine injection has been administered concurrently with morphine in several clinical trials of postoperative pain without evidence of adverse interactions. Do not mix ketorolac tromethamine and morphine in the same syringe.
There is no evidence in animal or human studies that ketorolac tromethamine induces or inhibits hepatic enzymes capable of metabolizing itself or other drugs.
Carcinogenesis, Mutagenesis, and Impairment of Fertility
An 18-month study in mice with oral doses of ketorolac tromethamine at 2 mg/kg/day (0.9 times the human systemic exposure at the recommended IM or IV dose of 30 mg qid, based on area-under-the-plasma concentration curve [AUC]), and a 24-month study in rats at 5 mg/kg/day (0.5 times the human AUC), showed no evidence of tumorigenicity.
Ketorolac tromethamine was not mutagenic in the Ames test, unscheduled DNA synthesis and repair, and in forward mutation assays. Ketorolac tromethamine did not cause chromosome breakage in the in vivo mouse micronucleus assay. At 1590 mcg/mL and at higher concentrations, ketorolac tromethamine increased the incidence of chromosomal aberrations in Chinese hamster ovarian cells.
Impairment of fertility did not occur in male or female rats at oral doses of 9 mg/kg (0.9 times the human AUC) and 16 mg/kg (1.6 times the human AUC) of ketorolac tromethamine, respectively.
Pregnancy
Pregnancy Category C. Reproduction studies have been performed during organogenesis using daily oral doses of ketorolac tromethamine at 3.6 mg/kg (0.37 times the human AUC) in rabbits and at 10 mg/kg (1.0 times the human AUC) in rats. Results of these studies did not reveal evidence of teratogenicity to the fetus. Doses of ketorolac tromethamine tablets at 1.5 mg/kg (0.14 times the human AUC), administered after gestation day 17, caused dystocia and higher pup mortality in rats. There are no adequate and well-controlled studies of ketorolac tromethamine in pregnant women. Ketorolac tromethamine should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Labor and Delivery
The use of ketorolac tromethamine is contraindicatedin labor and delivery because, through its prostaglandin synthesis inhibitory effect, it may adversely affect fetal circulation and inhibit uterine contractions, thus increasing the risk of uterine hemorrhage (see CONTRAINDICATIONS).
Lactation and Nursing
After a single administration of a 10 mg ketorolac tromethamine tablet to humans, the maximum milk concentration observed was 7.3 ng/mL and the maximum milk-to-plasma ratio was 0.037. After one day of dosing (qid), the maximum milk concentration was 7.9 ng/mL and the maximum milk-to-plasma ratio was 0.025. Because of the possible adverse effects of prostaglandin-inhibiting drugs on neonates, use in nursing mothers is CONTRAINDICATED.
Pediatric Use
The safety and effectiveness of single doses of ketorolac tromethamine injection have been established in pediatric patients between the ages of 2 and 16 years. Ketorolac tromethamine injection has been shown to be effective in the management of moderately severe acute pain that requires analgesia at the opioid level, usually in a postoperative setting. Safety and efficacy in pediatric patients below the age of 2 have not been established. Therefore, ketorolac tromethamine injection is not recommended in pediatric patients below the age of 2. The risk of bleeding was greater in those patients administered ketorolac tromethamine injection following tonsillectomy. Physicians should consider the increased risk of bleeding before deciding to administer ketorolac tromethamine injection in patients following tonsillectomy (see WARNINGS: Hemorrhage and Pediatrics and Tonsillectomy).
The risks identified in the adult population with ketorolac tromethamine injection use also apply to pediatric patients. Therefore, consult the CONTRAINDICATIONS, WARNINGS, PRECAUTIONS, and ADVERSE REACTIONS sections when prescribing ketorolac tromethamine injection to pediatric patients.
Geriatric Use (≥65 Years of Age)
Because ketorolac tromethamine may be cleared more slowly by the elderly (see CLINICAL PHARMACOLOGY) who are also more sensitive to the adverse effects of NSAIDs (see WARNINGS − Renal Effects), extra caution and reduced dosages (see DOSAGE AND ADMINISTRATION) must be used when treating the elderly with ketorolac tromethamine injection. The lower end of the ketorolac tromethamine injection dosage range is recommended for patients over 65 years of age and total daily dose is not to exceed 60 mg. The incidence and severity of gastrointestinal complications increases with increasing dose of, and duration of treatment with, ketorolac tromethamine.
KETOROLAC TROMETHAMINE ADVERSE REACTIONS
Adverse reaction rates increase with higher doses of ketorolac tromethamine. Practitioners should be alert for the severe complications of treatment with ketorolac tromethamine, such as G.I. ulceration, bleeding and perforation, postoperative bleeding, acute renal failure, anaphylactic and anaphylactoid reactions, and liver failure (see Boxed WARNING, WARNINGS, PRECAUTIONS, and DOSAGE AND ADMINISTRATION). These NSAID-related complications can be serious in certain patients for whom ketorolac tromethamine is indicated, especially when the drug is used inappropriately.
The Adverse Reactions Listed Below Were Reported In Clinical Trials As Probably Related To KETOROLAC TROMETHAMINE.
-
(Percentage of incidence in parentheses for those events reported in 3% or more patients)
Body as a Whole: edema (4%).
Cardiovascular: hypertension.
Dermatologic: pruritus, rash.
Gastrointestinal: nausea (12%), dyspepsia (12%), gastrointestinal pain (13%), diarrhea (7%), constipation, flatulence, gastrointestinal fullness, vomiting, stomatitis.
Hemic and Lymphatic: purpura.
Nervous System: headache (17%), drowsiness (6%), dizziness (7%), sweating.
Injection-site pain was reported by 2% of patients in multi-dose studies.
-
Body as a Whole: weight gain, fever, infections, asthenia.
Cardiovascular: palpitation, pallor, syncope.
Dermatologic: urticaria.
Gastrointestinal: gastritis, rectal bleeding, eructation, anorexia, increased appetite.
Hemic and Lymphatic: epistaxis, anemia, eosinophilia.
Nervous System: tremors, abnormal dreams, hallucinations, euphoria, extrapyramidal symptoms, vertigo, paresthesia, depression, insomnia, nervousness, excessive thirst, dry mouth, abnormal thinking, inability to concentrate, hyperkinesis, stupor.
Respiratory: dyspnea, pulmonary edema, rhinitis, cough.
Special Senses: abnormal taste, abnormal vision, blurred vision, tinnitus, hearing loss.
Urogenital: hematuria, proteinuria, oliguria, urinary retention, polyuria, increased urinary frequency.
The Following Adverse Events Were Reported From Postmarketing Experience.
Body as a Whole: hypersensitivity reactions such as anaphylaxis, anaphylactoid reaction, laryngeal edema, tongue edema (see Boxed WARNING, WARNINGS), angioedema, myalgia.
Cardiovascular: hypotension and flushing.
Dermatologic: Lyell’s syndrome, Stevens-Johnson syndrome, exfoliative dermatitis, maculo-papular rash, urticaria.
Gastrointestinal: peptic ulceration, G.I. hemorrhage, G.I. perforation (see Boxed WARNING, WARNINGS), melena, acute pancreatitis, hematemesis, esophagitis.
Hemic and Lymphatic: postoperative wound hemorrhage rarely requiring blood transfusion (see Boxed WARNING, WARNINGS and PRECAUTIONS), thrombocytopenia, leukopenia.
Hepatic: hepatitis, liver failure, cholestatic jaundice.
Nervous System: convulsions, psychosis, aseptic meningitis.
Respiratory: asthma, bronchospasm.
Urogenital: acute renal failure (see Boxed WARNING, WARNINGS), flank pain with or without hematuria and/or azotemia, nephritis, hyponatremia, hyperkalemia, hemolytic uremic syndrome.
OVERDOSAGE
Symptoms following acute NSAIDs overdoses are usually limited to lethargy, drowsiness, nausea, vomiting, and epigastric pain, which are generally reversible with supportive care. Gastrointestinal bleeding can occur. Hypertension, acute renal failure, respiratory depression and coma may occur, but are rare. Anaphylactoid reactions have been reported with therapeutic ingestion of NSAIDs, and may occur following an overdose.
Patients should be managed by symptomatic and supportive care following a NSAIDs overdose. There are no specific antidotes. Emesis and/or activated charcoal (60 g to 100 g in adults, 1 g/kg to 2 g/kg in children) and/or osmotic cathartic may be indicated in patients seen within 4 hours of ingestion with symptoms or following a large oral overdose (5 to 10 times the usual dose).
In controlled overdosage, daily doses of 360 mg of ketorolac tromethamine injection given for 5 days (three times the highest recommended dose), caused abdominal pain and peptic ulcers which healed after discontinuation of dosing. Metabolic acidosis has been reported following intentional overdosage.
Single overdoses of ketorolac tromethamine have been variously associated with abdominal pain, nausea, vomiting, hyperventilation, peptic ulcers and/or erosive gastritis and renal dysfunction which have resolved after discontinuation of dosing. Dialysis does not significantly clear ketorolac tromethamine from the blood stream.
KETOROLAC TROMETHAMINE DOSAGE AND ADMINISTRATION
IN ADULTS, THE COMBINED DURATION OF USE OF KETOROLAC TROMETHAMINE INJECTION AND KETOROLAC TROMETHAMINE TABLET IS NOT TO EXCEED 5 DAYS. IN ADULTS, THE USE OF KETOROLAC TROMETHAMINE TABLETS IS ONLY INDICATED AS CONTINUATION THERAPY TO KETOROLAC TROMETHAMINE INJECTION.
KETOROLAC TROMETHAMINE INJECTION
Adult Patients: Ketorolac tromethamine injection may be used as a single or multiple dose, on a regular or prn schedule for the management of moderately severe, acute pain that requires analgesia at the opioid level, usually in a postoperative setting. Hypovolemia should be corrected prior to the administration of ketorolac tromethamine (see WARNINGS − Renal Effects). Patients should be switched to alternative analgesics as soon as possible, but ketorolac tromethamine therapy is not to exceed 5 days.
When administering ketorolac tromethamine, the IV bolus must be given over no less than 15 seconds. The IM administration should be given slowly and deeply into the muscle. The analgesic effect begins in ~30 minutes with maximum effect in 1 to 2 hours after dosing IV or IM. Duration of analgesic effect is usually 4 to 6 hours.
Single-Dose Treatment: The following regimen should be limited to single administration use only.
Adult Patients:
IM Dosing:
-
-
IV Dosing:
-
-
Pediatric Patients (2 to 16 years of age): The pediatric population should receive only a single dose of ketorolac tromethamine injection as follows:
IM Dosing:
One dose of 1 mg/kg up to a maximum of 30 mg.
IV Dosing:
One dose of 0.5 mg/kg up to a maximum of 15 mg.
Multiple-Dose Treatment (IV or IM):
-
-
For breakthrough pain, do not increase the dose or the frequency of ketorolac tromethamine. Consideration should be given to supplementing these regimens with low doses of opioids “prn” unless otherwise contraindicated.
Pharmaceutical Information for Ketorolac Tromethamine Injection:
Ketorolac tromethamine should not be mixed in a small volume (e.g., in a syringe) with morphine sulfate, meperidine hydrochloride, promethazine hydrochloride or hydroxyzine hydrochloride; this will result in precipitation of ketorolac from solution.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
HOW SUPPLIED
Ketorolac Tromethamine Injection, USP for intramuscular and intravenous use is available in an iSecureTM sterile cartridge unit:
30 mg: 30 mg/mL, 1 mL iSecure sterile cartridge unit (with Luer Lock) boxes of:
10 NDC 21695-588-10
25 NDC 21695-588-10
Store at 20 to 25°C (68 to 77°F). [See USP Controlled Room Temperature.] Retain in carton until time of use.
PROTECT FROM LIGHT.
Revised: March, 2007
Directions for iSecure Syringe
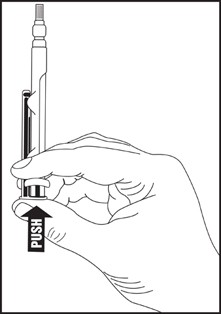
1. To release plunger rod, grasp syringe and depress rod until it releases from the syringe.
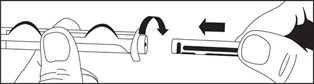
2. Attach plunger rod to the syringe barrel by inserting rod into the plunger end and turning clockwise.
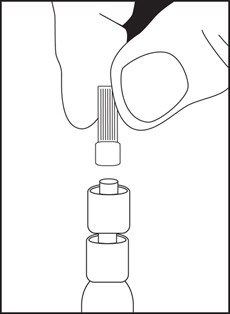
3. Remove luer tip cover. Attach needle or blunt cannula if applicable.
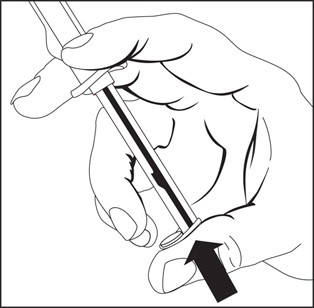
4. Expel air by pushing on the plunger rod. Do not touch the syringe tip. Administer Drug.
Note: To prevent needlestick injuries, needles and blunt cannulas should not be recapped, purposely bent, or broken by hand.
|
Printed in USA |
EN-1465 |
|
Hospira, Inc., Lake Forest, IL 60045 USA |
|
Packaged by:
Rebel Distributors Corp
Thousand Oaks, CA 91320
Principal Display Panel
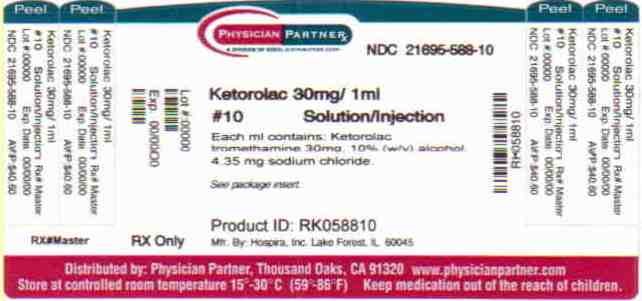
Ketorolac TromethamineKetorolac Tromethamine INJECTION, SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||