Ketorolac Tromethamine
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- KETOROLAC TROMETHAMINE DESCRIPTION
- CLINICAL PHARMACOLOGY
- CLINICAL STUDIES
- INDICATIONS & USAGE
- KETOROLAC TROMETHAMINE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- LABOR & DELIVERY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- KETOROLAC TROMETHAMINE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- SPL MEDGUIDE
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
BOXED WARNING
Ketorolac tromethamine tablets, a non-steroidal anti-inflammatory drug (NSAID), are indicated for the short-term (up to 5 days in adults), management of moderately severe acute pain that requires analgesia at the opioid level and only as continuation treatment following IV or IM dosing of ketorolac tromethamine, if necessary. The total combined duration of use of ketorolac tromethamine tablets and ketorolac tromethamine should not exceed 5 days.Ketorolac tromethamine tablets are not indicated for use in pediatric patients and they are NOT indicated for minor or chronic painful conditions. Increasing the dose of ketorolac tromethamine tablets beyond a daily maximum of 40 mg in adults will not provide better efficacy but will increase the risk of developing serious adverse events.
GASTROINTESTINAL RISK
-
● Ketorolac tromethamine, including ketorolac tromethamine tablets can cause peptic ulcers, gastrointestinal bleeding and/or perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Therefore, ketorolac tromethamine is CONTRAINDICATED in patients with active peptic ulcer disease, in patients with recent gastrointestinal bleeding or perforation, and in patients with a history of peptic ulcer disease or gastrointestinal bleeding. Elderly patients are at greater risk for serious gastrointestinal events (see WARNINGS).
-
● NSAIDs may cause an increased risk of serious cardiovascular thrombotic events, myocardial infarction, and stroke, which can be fatal. This risk may increase with duration of use. Patients with cardiovascular disease or risk factors for cardiovascular disease may be at greater risk (see WARNINGS and CLINICAL STUDIES).
-
● Ketorolac tromethamine is contraindicated for the treatment of peri-operative pain in the setting of coronary artery bypass graft (CABG) surgery (see WARNINGS).
-
● Ketorolac tromethamine is CONTRAINDICATED in patients with advanced renal impairment and in patients at risk for renal failure due to volume depletion (see WARNINGS).
-
● Ketorolac tromethamine inhibits platelet function and is, therefore, CONTRAINDICATED in patients with suspected or confirmed cerebrovascular bleeding, patients with hemorrhagic diathesis, incomplete hemostasis and those at high risk of bleeding (see WARNINGS and PRECAUTIONS).
RISK DURING LABOR AND DELIVERY
-
● The use of ketorolac tromethamine in labor and delivery is contraindicated because it may adversely affect fetal circulation and inhibit uterine contractions. The use of ketorolac tromethamine is contraindicated in nursing mothers because of the potential adverse effects of prostaglandin-inhibiting drugs on neonates.
-
● Ketorolac tromethamine is CONTRAINDICATED in patients currently receiving aspirin or NSAIDs because of the cumulative risk of inducing serious NSAID-related side effects.
-
● Dosage should be adjusted for patients 65 years or older, for patients under 50 kg (110 lbs) of body weight (see DOSAGE AND ADMINISTRATION) and for patients with moderately elevated serum creatinine (see WARNINGS).
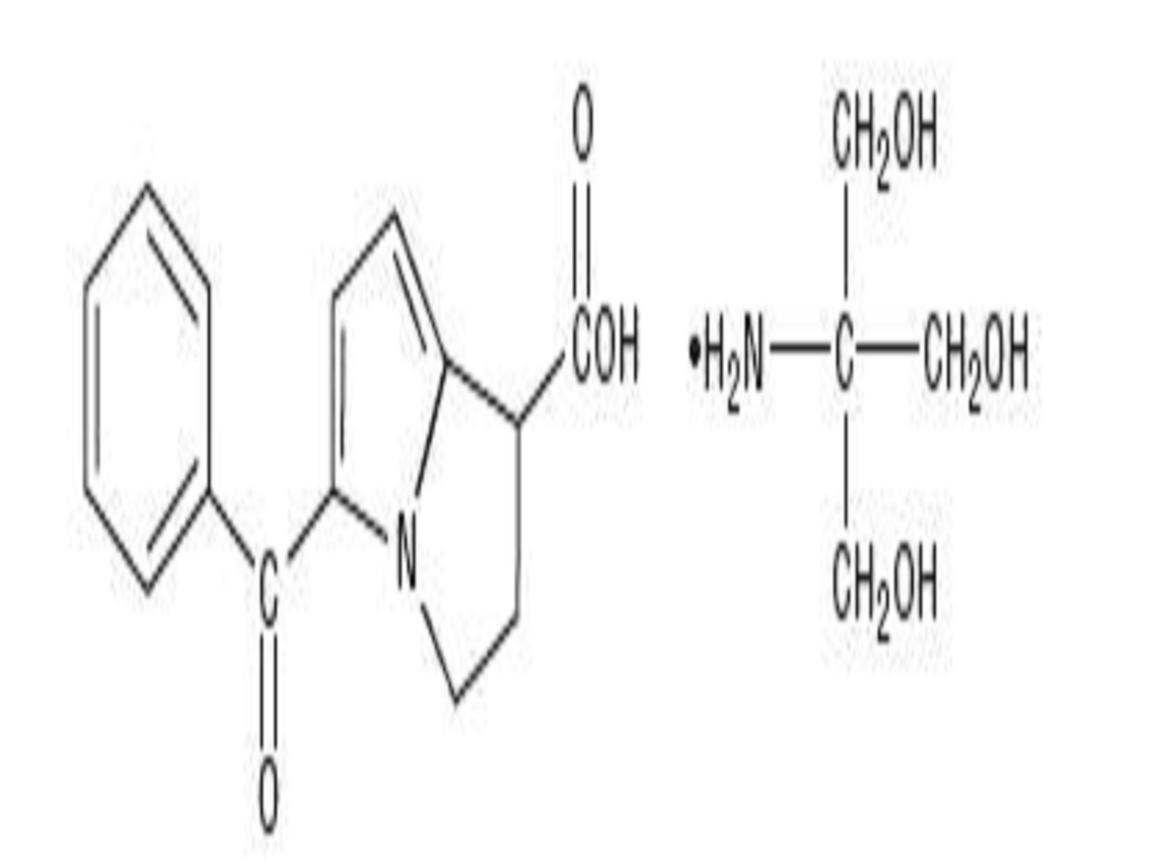
KETOROLAC TROMETHAMINE DESCRIPTION
CLINICAL PHARMACOLOGY
PharmacodynamicsPharmacokinetics
Comparison of IV, IM, and Oral Pharmacokinetics
Linear Kinetics
Absorption
Distribution
Metabolism
Excretion
Accumulation
Kinetics in Special Populations
Geriatric Patients
Pediatric Patients
Renal Insufficiency
Hepatic Insufficiency
Race
*#*#
*
*
IV Administration
CLINICAL STUDIES
Adult PatientsPediatric Patients
INDICATIONS & USAGE
Acute Pain in Adult Patients
KETOROLAC TROMETHAMINE CONTRAINDICATIONS
WARNINGS
Gastrointestinal EffectsRisk of Ulceration, Bleeding, and Perforation
Hemorrhage
Renal Effects
Impaired Renal Function
Anaphylactoid Reactions
Cardiovascular Effects
Cardiovascular Thrombotic Events
Hypertension
Congestive Heart Failure and Edema
Skin Reactions
Pregnancy
PRECAUTIONS
GeneralHepatic Effect
Hematologic Effect
Preexisting Asthma
INFORMATION FOR PATIENTS
LABORATORY TESTS
DRUG INTERACTIONS
Warfarin, Digoxin, Salicylate, and Heparin
Aspirin
Diuretics
Probenecid
Lithium
Methotrexate
ACE Inhibitors/Angiotensin II Receptor Antagonists
Antiepileptic Drugs
Psychoactive Drugs
Pentoxifylline
Nondepolarizing Muscle Relaxants
Selective Serotonin Reuptake Inhibitors (SSRIs)
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Teratogenic EffectsPregnancy category C
Nonteratogenic Effects
LABOR & DELIVERY
Effects on Fertility
NURSING MOTHERS
PEDIATRIC USE
GERIATRIC USE
(65 Years of Age)KETOROLAC TROMETHAMINE ADVERSE REACTIONS
Body as a Whole:
Cardiovascular
Dermatologic:
Gastrointestinal:
Hemic and Lymphatic:
Metabolic and Nutritional:
Nervous System:
Reproductive, female:
Respiratory:
Special Senses:
Urogenital:
Body as a Whole:
Cardiovascular:
Dermatologic:
Gastrointestinal:
Hemic and Lymphatic:
Metabolic and Nutritional:
Nervous System:
Respiratory:
Special Senses
Urogenital:
Postmarketing Surveillance Study
OVERDOSAGE
Symptoms and SignsTreatment
DOSAGE & ADMINISTRATION
Carefully consider the potential benefits and risks of ketorolac tromethamine and other treatment options before deciding to use ketorolac tromethamine. Use the lowest effective dose for the shortest duration consistent with individual patient treatment goals. In adults, the combined duration of use of IV or IM dosing of ketorolac tromethamine and ketorolac tromethamine tablets is not to exceed 5 days. In adults, the use of ketorolac tromethamine tablets is only indicated as continuation therapy to IV or IM dosing of ketorolac tromethamine.Transition from IV or IM dosing of ketorolac tromethamine (single- or multiple-dose) to multiple-dose ketorolac tromethamine tablets:
Note:
HOW SUPPLIED
SPL MEDGUIDE
Medication Guide for Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)Rx only
What is the most important information I should know about medicines called Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)?
NSAID medicines may increase the chance of a heart attack or stroke that can lead to death.
-
● with longer use of NSAID medicines
-
● in people who have heart disease
NSAID medicines can cause ulcers and bleeding in the stomach and intestines at any time during treatment. Ulcers and bleeding:
-
● can happen without warning symptoms
-
● may cause death
-
● taking medicines calledcorticosteroidsandanticoagulants
-
● longer use
-
● smoking
-
● drinking alcohol
-
● older age
-
● having poor health
-
● exactly as prescribed
-
● at the lowest dose possible for your treatment
-
● for the shortest time needed
-
● different types of arthritis
-
● menstrual cramps and other types of short-term pain
Do not take an NSAID medicine:
-
● if you had an asthma attack, hives, or other allergic reaction with aspirin or any other NSAID medicine
-
● for pain right before or after heart bypass surgery
-
● about all of your medical conditions.
-
● about all of the medicines you take. NSAIDs and some other medicines can interact with each other and cause serious side effects. Keep a list of your medicines to show to your healthcare provider and pharmacist.
-
● if you are pregnant. NSAID medicines should not be used by pregnant women late in their pregnancy.
-
● if you are breastfeeding. Talk to your doctor.
Get emergency help right away if you have any of the following symptoms:
-
● shortness of breath or trouble breathing
-
● chest pain
-
● weakness in one part or side of your body
-
● slurred speech
-
● swelling of the face or throat
-
● nausea
-
● more tired or weaker than usual
-
● itching
-
● your skin or eyes look yellow
-
● stomach pain
-
● flu-like symptoms
-
● vomit blood
-
● there is blood in your bowel movement or it is black and sticky like tar
-
● unusual weight gain
-
● skin rash or blisters with fever
-
● swelling of the arms and legs, hands and feet
Other information about Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)
-
● Aspirin is an NSAID medicine but it does not increase the chance of a heart attack. Aspirin can cause bleeding in the brain, stomach, and intestines. Aspirin can also cause ulcers in the stomach and intestines.
-
● Some of these NSAID medicines are sold in lower doses without a prescription (over-the-counter). Talk to your healthcare provider before using over-the-counter NSAIDs for more than 10 days.
**Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION


Ketorolac TromethamineKetorolac Tromethamine TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!