Jay-Phyl
JayMac Pharmaceuticals LLC
Great Southern Laboratories
Jay-Phyl Syrup
FULL PRESCRIBING INFORMATION: CONTENTS*
- Jay-Phyl Syrup
- CLINICAL PHARMACOLOGY
- JAY-PHYL INDICATIONS AND USAGE:
- JAY-PHYL CONTRAINDICATIONS:
- WARNINGS
- PRECAUTIONS:
- Drug Interactions:
- Carcinogenesis, Mutagenesis, Impairment of Fertility:
- Pregnancy: Teratogenic effects- Pregnancy Category C.
- Nursing Mothers:
- Pediatric Use:
- JAY-PHYL ADVERSE REACTIONS
- OVERDOSAGE
- JAY-PHYL DOSAGE AND ADMINISTRATION:
- HOW SUPPLIED:
- STORAGE:
- PRODUCT PACKAGING:
FULL PRESCRIBING INFORMATION
Jay-Phyl Syrup
Bronchodilator/ExpectorantRx Only
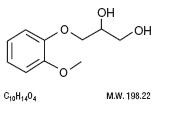
DESCRIPTION:
Inactive ingredients:

CLINICAL PHARMACOLOGY
INDICATIONS AND USAGE:
CONTRAINDICATIONS:
WARNINGS
PRECAUTIONS:
General:Drug Interactions:
Carcinogenesis, Mutagenesis, Impairment of Fertility:
Pregnancy: Teratogenic effects- Pregnancy Category C.
Nursing Mothers:
Pediatric Use:
JAY-PHYL ADVERSE REACTIONS
Gastrointestinal:
Central Nervous System:
Cardiovascular:
Respiratory: tachypnea.
Renal:
Other:
OVERDOSAGE
Signs and Symptoms:Treatment:
DOSAGE AND ADMINISTRATION:
Use in Children:
HOW SUPPLIED:
Rx Only
WARNING: KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
IN CASE OF ACCIDENTAL OVERDOSE, SEEK PROFESSIONAL ASSISTANCE OR
CONTACT A POISON CONTROL CENTER IMMEDIATELY.
STORAGE:
ooooPRODUCT PACKAGING:
Principal Display Panel and Side Panel for 15 mL Label:
NDC 64661-814-15
Jay-Phyl Syrup
Each 5 mL contains:
Dyphylline............. 100 mg
Guaifenesin........... 50 mg
Sugar and Alcohol Free
Rx Only
1/2 fl oz (15 mL)
DOSAGE AND ADMINISTRATION:
Adults:
oooo
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
IN CASE OF ACCIDENTAL OVERDOSE, CONTACT A POISON CONTROL
CENTER AND SEEK PROFESSIONAL ASSISTANCE IMMEDIATELY.
Manufactured for:
PROFESSIONAL SAMPLE
NOT FOR RESALE

Jay-PhylDyphylline and Guaifenesin SYRUP
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!