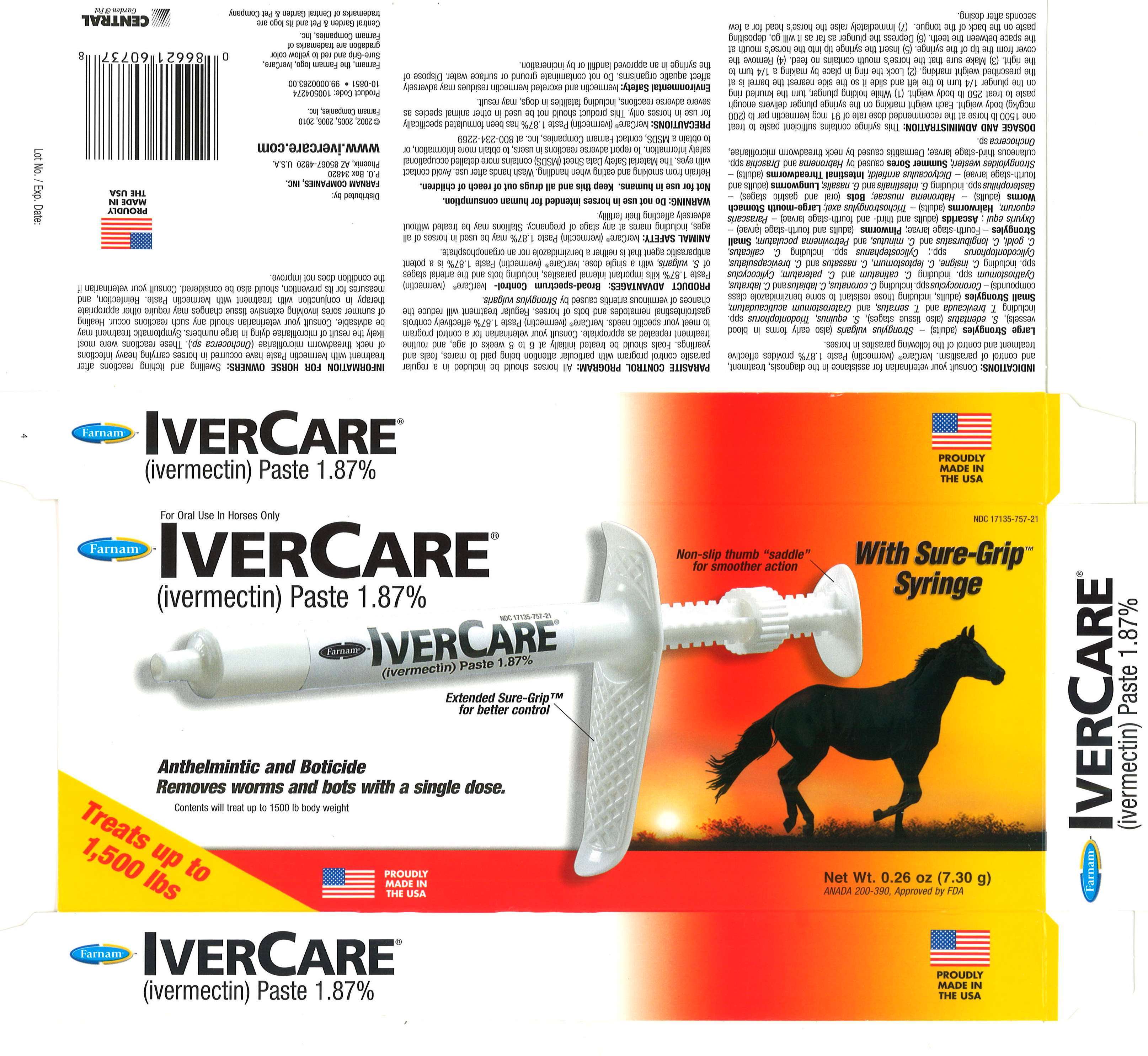
IverCare
Farnam Companies, Inc.
Med-Pharmex, Inc
Ivermectin Paste
FULL PRESCRIBING INFORMATION
Uses
ANADA 200-390, Approved by the FDA
NDC 17135-757-21
IVERCARE (ivermectin)
Paste 1.87% - Anthelmintic and Boticide
Removes worms and bots with a single dose.
Contents will treat up to 1500 ib body weight.
For Oral Use in Horses Only
Each Syringe Contains0.26 OZ (7.30 g) IVERCARE (ivermectin)
Paste 1.87%
Net Wt: 0.26 oz (7.30g)
INDICATIONS: Consult your veterinarian for assistance in the diagnosis, treatment, and control of parasitism. IverCare (ivermectin) Paste 1.87% provides effective treatment and control of the following parasites in horses.
Large Strongyles (adults) – Strongylus vulgaris (also early forms in blood vessels), S. edentatus (also tissue stages), S. equinus, Triodontophorus spp. including T. brevicauda and T. serratus and Craterostomum acuticaudatum; Small Strongyles (adults, including those resistant to some benzimidazole class compounds) – Coronocyclus spp. including C. coronatus, C. labiatus and C. labratus, Cyathostomum spp. including C. catinatum and C. pateratum, Cylicocyclus spp. including C. insigne, C. leptostomum, C. nassatus and C. brevicapsulatus, Cylicodontophorus spp., Cylicostephanus spp. including C. calicatus, C. goldi, C. longibursatus and C. minutus, and Petrovinema poculatum; Small Strongyles – Fourth-stage larvae; Pinworms (adults and fourth-stage larvae) – Oxyuris equi; Ascarids (adults and third- and fourth-stage larvae) – Parascaris equorum; Hairworms (adults) – Trichostrongylus axei; Large-mouth Stomach Worms (adults) – Habronema muscae; Bots (oral and gastric stages) – Gasterophilus spp. including G. intestinalis and G. nasalis; Lungworms (adults and fourth-stage larvae) – Dictyocaulus arnfieldi; Intestinal Threadworms (adults) – Strongyloides westeri; Summer Sores caused by Habronema and Draschia spp. cutaneous third-stage larvae; Dermatitis caused by neck threadworm microfilariae, Onchocerca sp.
DOSAGE AND ADMINISTRATION: This syringe contains sufficient paste to treat one 1500 lb horse at the recommended dose rate of 91 mcg ivermectin per lb (200 mcg/kg) body weight. Each weight marking on the syringe plunger delivers enough paste to treat 250 lb body weight. (1) While holding plunger, turn the knurled ring on the plunger 1/4 turn to the left and slide it so the side nearest the barrel is at the prescribed weight marking. (2) Lock the ring in place by making a 1/4 turn to the right. (3) Make sure that the horse's mouth contains no feed. (4) Remove the cover from the tip of the syringe. (5) Insert the syringe tip into the horse's mouth at the space between the teeth. (6) Depress the plunger as far as it will go, depositing paste on the back of the tongue. (7) Immediately raise the horse's head for a few seconds after dosing.
PARASITE CONTROL PROGRAM: All horses should be included in a regular parasite control program with particular attention being paid to mares, foals and yearlings. Foals should be treated initially at 6 to 8 weeks of age, and routine treatment repeated as appropriate. Consult your veterinarian for a control program to meet your specific needs. IverCare (ivermectin) Paste 1.87% effectively controls gastrointestinal nematodes and bots of horses. Regular treatment will reduce the chances of verminous arteritis caused by Strongylus vulgaris.
PRODUCT ADVANTAGES: Broad-spectrum Control– IverCare (ivermectin) Paste 1.87% kills important internal parasites, including bots and the arterial stages of S. vulgaris, with a single dose. IverCare (ivermectin) Paste 1.87% is a potent antiparasitic agent that is neither a benzimidazole nor an organophosphate.
ANIMAL SAFETY: IverCare (ivermectin) Paste 1.87% may be used in horses of all ages, including mares at any stage of pregnancy. Stallions may be treated without adversely affecting their fertility.
WARNING: Do not use in horses intended for human consumption.
Not for use in humans. Keep this and all drugs out of reach of children.
Refrain from smoking and eating when handling. Wash hands after use. Avoid contact with eyes. The Material Safety Data Sheet (MSDS) contains more detailed occupational safety information. To report adverse reactions in users, to obtain more information, or to obtain a MSDS, contact Farnam Companies, Inc. at 800-234-2269.
PRECAUTIONS: IverCare (ivermectin) Paste 1.87% has been formulated specifically for use in horses only. This product should not be used in other animal species as severe adverse reactions, including fatalities in dogs, may result.
Environmental Safety: Ivermectin and excreted ivermectin residues may adversely affect aquatic organisms. Do not contaminate ground or surface water. Dispose of the syringe in an approved landfill or by incineration.
INFORMATION FOR HORSE OWNERS: Swelling and itching reactions after treatment with Ivermectin Paste have occurred in horses carrying heavy infections of neck threadworm microfilariae (Onchocerca sp.) . These reactions were most likely the result of microfilariae dying in large numbers. Symptomatic treatment may be advisable. Consult your veterinarian should any such reactions occur. Healing of summer sores involving extensive tissue changes may require other appropriate therapy in conjunction with treatment with Ivermectin Paste. Reinfection, and measures for its prevention, should also be considered. Consult your veterinarian if the condition does not improve.
2010 Farnam Companies, Inc. 10-0855 99 0000448.00 rev 5/10
IverCare is a registered trademark of Farnam Companies, Inc.
IverCare
(ivermectin) Paste 1.87%
Anthelmintic and Boticide

IverCareIvermectin PASTE
| |||||||||||||||||||||||||||||||||||||||||||||||||