I.V. Prep Antiseptic Wipe
I.V. Prep Antiseptic Wipe
FULL PRESCRIBING INFORMATION: CONTENTS*
- DRUG FACTS
- ACTIVE INGREDIENTS (IN EACH PACKET)
- PURPOSE
- USES
- WARNINGS
- DIRECTIONS
- INACTIVE INGREDIENTS
- QUESTION OR COMMENTS?
FULL PRESCRIBING INFORMATION
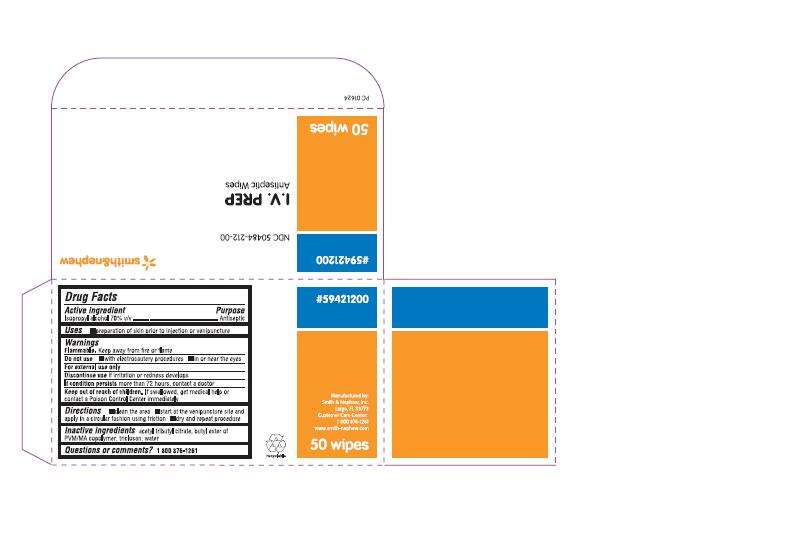
DRUG FACTS
ACTIVE INGREDIENTS (IN EACH PACKET)
Isopropyl Alcohol 70% V/V
PURPOSE
Antiseptic
USES
Preparation of skin prior to injection or venipuncture
WARNINGS
- For external use only
- Flammable. Keep away from fire or flame
-
Do not use
- with electrocautery procedures
- in or near the eyes
- Discontinue use if irritation or redness develops
- If condition persists more than 72 hours, contact a doctor
- Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center immediately
DIRECTIONS
- clean the area
- start at the venipuncture site and apply in a circular fashion using friction
- dry and repeat procedure
INACTIVE INGREDIENTS
acetyl tributyl citrate, butyl ester of PVM/MA copolymer, triclosan, water
QUESTION OR COMMENTS?
Smith & Nephew, Inc
Largo, FL 33773
Customer Care Center:
1 800 876-1261
www.smith-nephew.com
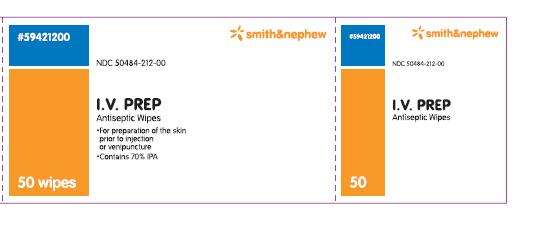
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - BOX OF 50 WIPES (Front)
#59421200
NDC 50484-212-00
I.V. PREP
Antiseptic Wipes
- for preparation of the skin prior to injection or venipuncture
- contains 70% IPA
50 Wipes
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - BOX OF 50 WIPES (Back)
#59421200
NDC 50484-212-00
I.V. PREP
Antiseptic Wipes
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 1 Packet
#59421200
NDC 50484-212-00
I.V. PREP
Antiseptic Wipes
- for preparation of the skin prior to injection or venipuncture
- contains 70% IPA
1 Wipe

I.V. Prep Antiseptic WipeISOPROPYL ALCOHOL SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||