Isoplate
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use Isoplate™ Solution Platelet Additive Solution [PAS-F] safely and effectively. See full prescribing information for Isoplate Solution Platelet Additive Solution [PAS-F]. Isoplate SolutionPlatelet Additive Solution [PAS-F] Initial U.S. Approval: 2013INDICATIONS AND USAGEIsoplate Solution Platelet Additive Solution [PAS-F] is an isotonic solution to replace a portion of the plasma to store Platelet Pheresis, Leukocytes Reduced PAS products collected using a hyperconcentrated collection on Terumo BCT's Trima Accel® System. The solution should never be infused directly to the patient. Platelet Pheresis, Leukocytes Reduced Platelet Additive Solution [PAS] products are stored in a mix of 65% Isoplate and 35% plasma. Platelets in Isoplate Solution can be stored at a concentration range of 700-2100 × 106/mL for up to 5 days at 20-24°C with continuous agitation in the EXCEL® container. (1)DOSAGE AND ADMINISTRATIONIsoplate Solution Platelet Additive Solution [PAS-F] may only be used with the Trima Accel automated blood cell separator device. For full instructions on the use of Isoplate Solution Platelet Additive Solution [PAS-F] with the Trima Accel see the Trima Accel Operator's Manual. (2) Directions for Connecting the Isoplate Solution Container to the Trima Accel System At the prompt to connect the platelet additive solution to the Trima Accel tubing set: Remove the overwrap by pulling down at notch and remove solution container. Before use, perform the following checks: Check for leaks by squeezing the bag firmly. If leaks are found, discard solution. Ensure the solution is the Isoplate Solution Platelet Additive Solution [PAS-F] and is within the expiration date. Inspect the solution for cloudiness, haze, or particulate matter. Suspect container should not be used. Remove the protective cap from the port on the Isoplate™ Solution Platelet Additive Solution [PAS-F] container. Connect the Isoplate container to the Trima Accel® set using aseptic technique and hang the solution. Proceed per the Trima Accel Operator's Manual. DOSAGE FORMS AND STRENGTHS 500 mL bag (3) CONTRAINDICATIONSThere are no known contraindications. (4)WARNINGS AND PRECAUTIONS Isoplate Solution Platelet Additive Solution [PAS-F] is NOT FOR DIRECT INTRAVENOUS INFUSION. (5) Verify that the Isoplate Solution Platelet Additive Solution [PAS-F] has been securely attached to the platelet additive solution line on the Trima Accel disposable tubing set using aseptic technique. (5) Multiple transfusions of Isoplate-stored platelets may lead to overdosage of potassium, magnesium and gluconate (5, 10). Side Effects Isoplate Solution Platelet Additive Solution [PAS-F] is added to leukoreduced hyperconcentrated platelets after the Trima Accel apheresis procedure is complete. Isoplate Solution Platelet Additive Solution [PAS-F] is not expected to cause adverse events other than those normally associated with platelet transfusion. (6) To report SUSPECTED ADVERSE REACTIONS, contact Terumo BCT, Inc at 1-877-339-4228 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONS Isoplate Solution Platelet Additive Solution [PAS-F] is used as a storage solution for hyperconcentrated platelets and it is not intended for direct intravenous infusion. There are no known drug interactions in its use as a platelet storage solution. (7)
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 ISOPLATE INDICATIONS AND USAGE
- 2 ISOPLATE DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 ISOPLATE CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 ISOPLATE ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 9 DRUG ABUSE AND DEPENDENCE
- 10 OVERDOSAGE
- 11 ISOPLATE DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 14 CLINICAL STUDIES
- 15 REFERENCES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
Isoplate™ Solution Platelet Additive Solution [PAS-F] is an isotonic solution to replace a portion of the plasma to store Platelet Pheresis, Leukocytes Reduced PAS products collected using a hyperconcentrated collection on Terumo BCT's Trima Accel® System. The solution should never be infused directly to the patient. Platelet Pheresis, Leukocytes Reduced Platelet Additive Solution [PAS] products are stored in a mix of 65% Isoplate and 35% plasma. Platelets in Isoplate Solution can be stored at a concentration range of 700-2100 × 106/mL for up to 5 days at 20-24°C with continuous agitation in the EXCEL® container.
2 DOSAGE AND ADMINISTRATION
2.1 General Dosing Information
Isoplate Solution Platelet Additive Solution [PAS-F] may only be used with the Trima Accel automated blood cell separator device. For full instructions on the use of Isoplate Solution Platelet Additive Solution [PAS-F] with the Trima Accel see the Trima Accel Operator's Manual.
2.2 Directions for Connecting the Isoplate™ Solution Container to the Trima Accel® System
At the prompt to connect the platelet additive solution to the Trima Accel tubing set:
- Remove the overwrap by pulling down at notch and remove solution container.
- Before use, perform the following checks:
- Check for leaks by squeezing the bag firmly. If leaks are found, discard solution.
- Ensure the solution is the Isoplate Solution Platelet Additive Solution [PAS-F] and is within the expiration date.
- Inspect the solution for cloudiness, haze, or particulate matter. Suspect container should not be used.
- Remove the protective cap from the port on the Isoplate Solution Platelet Additive Solution [PAS-F] container.
- Connect the Isoplate container to the Trima Accel set using aseptic technique and hang the solution.
- Proceed per the Trima Accel Operator's Manual.
3 DOSAGE FORMS AND STRENGTHS
Isoplate Solution Platelet Additive Solution [PAS-F] in the EXCEL® Container
| NDC | REF | Volume |
|---|---|---|
| 14537-408-50 | L7771 | 500 mL |
4 CONTRAINDICATIONS
There are no known contraindications.
5 WARNINGS AND PRECAUTIONS
- Isoplate™ Solution Platelet Additive Solution [PAS-F] is NOT FOR DIRECT INTRAVENOUS INFUSION.
- Multiple transfusions of Isoplate-stored platelets may lead to overdosage of potassium, magnesium and gluconate [See Overdosage (10) ].
- Inspect each container. Read the label. Ensure solution is the one ordered and is within the expiration date.
- Invert container and carefully inspect the solution in good light for cloudiness, haze, or particulate matter. Any container which is suspect should not be used.
- Use only if solution is clear and container and seals are intact.
- Do not reuse. Discard unused or partially used Isoplate Solution Platelet Additive Solution [PAS-F].
- Protect from sharp objects.
- Verify that the Isoplate Solution Platelet Additive Solution [PAS-F] has been securely attached to the platelet additive solution line on the Trima Accel® disposable tubing set using aseptic technique.
6 ADVERSE REACTIONS
Isoplate Solution Platelet Additive Solution [PAS-F] is added to Platelet Pheresis, Leukocytes Reduced PAS products after completion of a hyperconcentrated Trima Accel apheresis collection procedure. It is not for direct intravenous infusion. Isoplate Solution Platelet Additive Solution [PAS-F] is not expected to cause adverse events other than those normally associated with platelet transfusion.
6.1 Clinical Trials Experience
No adverse reactions were reported in subjects infused with a small volume (< 10 mL) of radiolabeled platelets that had been stored for 5 days in 65% Isoplate Solution Platelet Additive Solution [PAS-F], but rinsed prior to infusion.
7 DRUG INTERACTIONS
Isoplate Solution Platelet Additive Solution [PAS-F] is used as a storage solution for hyperconcentrated platelets and it is not intended for direct intravenous infusion. There are no known drug interactions in its use as a platelet storage solution.
9 DRUG ABUSE AND DEPENDENCE
Isoplate Solution Platelet Additive Solution [PAS-F] is used as a storage solution for leukoreduced hyperconcentrated platelets and has no pharmacological effect.
10 OVERDOSAGE
Multiple transfusions of Isoplate-stored platelets may lead to overdosage of potassium, magnesium and gluconate. Monitor changes in electrolyte concentration and acid-base balance when multiple transfusions of Isoplate-stored platelets are administered. In the event of overdose, discontinue transfusion immediately and institute appropriate corrective treatment.
The signs and symptoms of potassium overdose include paresthesias of the extremities, areflexia, muscular or respiratory paralysis, mental confusion, weakness, hypotension, cardiac arrhythmias, heart block, electrocardiographic abnormalities and cardiac arrest. Treatment of hyperkalemia includes the following;
- Dextrose Injection, USP 10% or 25%, containing 10 units of crystalline insulin per 20 grams of dextrose administered intravenously, 300 to 500 mL/hour.
- Absorption and exchange of potassium using sodium or ammonium cycle cation exchange resin, orally and as retention enema.
- Hemodialysis and peritoneal dialysis. The use of potassium-containing foods or medications must be eliminated. However, in cases of digitalization, too rapid a lowering of plasma potassium concentration can cause digitalis toxicity.
Gluconate overdose may cause metabolic alkalosis. Treatment of metabolic alkalosis may include administration of an acidifying agent such as ammonium chloride.
Signs of magnesium overdose include flushing, sweating, hypotension, depressed reflexes, flaccid paralysis, hypothermia, circulatory collapse, cardiac and CNS depression proceeding to respiratory paralysis. Disappearance of the patellar reflex is a useful clinical sign to detect the onset of magnesium overdose. In the event of overdosage artificial ventilation may be required until a calcium salt can be injected intravenously to antagonize the effects of magnesium.
11 DESCRIPTION
Isoplate™ Solution Platelet Additive Solution [PAS-F] is sterile, nonpyrogenic, and contains no bacteriostatic or antimicrobial agents. Isoplate Solution Platelet Additive Solution [PAS-F] is indicated as a platelet additive solution for the 5 day storage of Platelet Pheresis, Leukocytes Reduced PAS products collected using a hyperconcentrated collection on Terumo BCT's Trima Accel® System. Platelets in Isoplate Solution Platelet Additive Solution [PAS-F] can be stored at a concentration range of 700-2100 × 106/mL for up to 5 days at 20-24 °C with agitation.
The formulas of the active ingredients are provided in Table 1.
| Ingredients | Molecular Formula | Molecular Weight |
|---|---|---|
| Sodium Chloride USP | NaCl | 58.44 |
| Sodium Acetate Trihydrate USP | CH3COONa•3H2O | 136.08 |
| Potassium Chloride USP | KCl | 74.55 |
| Magnesium Chloride Hexahydrate USP | MgCl2•6H2O | 203.30 |
| Dibasic Sodium Phosphate Heptahydrate USP | Na2HPO4•7H2O | 268.07 |
| Monobasic Potassium Phosphate NF | KH2PO4 | 136.09 |
| Sodium Gluconate USP |
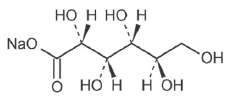
|
218.14 |
Each 100 mL of Isoplate Solution Platelet Additive Solution [PAS-F] contains: Sodium Chloride USP 0.53 g; Sodium Gluconate USP 0.5 g; Sodium Acetate Trihydrate USP 0.37 g; Potassium Chloride USP 0.037 g; Magnesium Chloride Hexahydrate USP 0.03 g; Dibasic Sodium Phosphate Heptahydrate USP 0.012 g; Monobasic Potassium Phosphate NF 0.00082 g; Water for Injection USP qs
pH may be adjusted with Glacial Acetic Acid USP or Sodium Hydroxide NF pH: 7.4 (7.0-7.8)
Calculated Osmolarity: 295 mOsmol/liter
Concentration of Electrolytes (mEq/liter): Sodium 141; Potassium 5; Magnesium 3; Chloride 98; Phosphate (HPO=4) 1 (0.5 mmole P/liter); Acetate (CH3COO–) 27; Gluconate (HOCH2(CHOH)4COO–) 23
Not made with natural rubber latex, PVC, or Di(2-ethylhexyl)phthalate (DEHP).
The plastic container is made from a multilayered film. It contains no plasticizers and exhibits virtually no leachables. The solution contact layer is a rubberized copolymer of ethylene and propylene. The container is nontoxic and biologically inert. The container-solution unit is a closed system and is not dependent upon entry of external air during administration. The container is overwrapped to provide protection from the physical environment and to provide an additional moisture barrier when necessary.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Isoplate Solution Platelet Additive Solution [PAS-F] contains the following key components to maintain platelet function:
- Sodium chloride for osmolarity
- Acetate to fuel platelet metabolism
- Gluconate or phosphate for buffering
- Magnesium and potassium to reduce platelet activation1,2
Isoplate Solution Platelet Additive Solution [PAS-F] is used as a storage solution for leukoreduced hyperconcentrated platelets and it is not for direct intravenous infusion. This solution has no pharmacological effect; the solution provides the appropriate components for platelet function while allowing for a lower volume of plasma in the platelet product during storage.
14 CLINICAL STUDIES
In Vivo radiolabeled recovery and survival
A paired study was completed to verify that in vivo radiolabeled recovery and survival of hyperconcentrated leukocyte reduced platelets collected on the Trima Accel System, diluted in Isoplate Solution Platelet Additive Solution [PAS-F], and stored for five days (Test) meet FDA acceptance criteria in comparison with fresh autologous platelets (Control). Table 2 summarizes the in vivo radiolabeled platelet recovery and survival data.
| Recovery | Survival | |||||
|---|---|---|---|---|---|---|
| Test | Control | Test/Control | Test | Control | Test/Control | |
| % | % | % | Days | Days | % | |
| Average | 51.1 | 60.2 | 85 | 6.6 | 8.7 | 76 |
| St. Dev. | 10.9 | 10.2 | 10 | 1.2 | 0.9 | 12 |
| Min | 32.6 | 40.4 | 66 | 4.5 | 6.4 | 52 |
| Max | 84.1 | 82.8 | 102 | 8.8 | 10.0 | 104 |
The primary outcomes for this study were:
| Recovery: | Test minus 66% Control is equal to or greater than zero with one-sided 97.5% confidence limit |
| Survival: | Test minus 58% Control is equal to or greater than zero with one-sided 97.5% confidence limit |
Both primary outcomes were met for hyperconcentrated leukocyte reduced platelets collected by apheresis and stored in Isoplate™ Solution Platelet Additive Solution [PAS-F].
In Vitro Platelet Quality Study
A paired study was completed to verify that in vitro platelet quality (functional assays) of hyperconcentrated leukocyte reduced platelets collected on the Trima Accel® System, diluted in Isoplate Solution Platelet Additive Solution [PAS-F], and stored for five days (Test) meet FDA acceptance criteria in comparison to plasmastored platelets (Control).
Table 3 summarizes the in vitro platelet quality data.
| Functional Assay | Isoplate Stored Apheresis Platelets (Test) Average (Standard Deviation) |
Plasma Stored Apheresis Platelets (Control) Average (Standard Deviation) |
|---|---|---|
| pH | 7.4 (0.2) | 7.5 (0.1) |
| CD62 Expression; P-Selectin (%) | 22.8 (15.6) | 15.0 (9.8) |
| Morphology Score (Max Score 400) | 289 (49) | 292 (47) |
| Hypotonic Shock Response (%) | 53.3 (12.4) | 55.9 (10.9) |
| Extent of Shape Change (%) | 23.2 (5.0) | 25.0 (6.0) |
The primary outcome for this study was:
| pH: | 95% or more of test units will have a pH (22°C) greater than 6.2 with a one sided confidence interval of 95% |
All 66 platelet products collected in this study had pH > 6.2 therefore the primary outcome for pH was met for hyperconcentrated platelets collected by apheresis and stored in Isoplate Solution Platelet Additive Solution [PAS-F].
15 REFERENCES
- Gulliksson H. Platelet storage media. Transfus Apher Sci 2001;24:241-4.
- Ringwald J, Zimmermann R, Eckstein R. The new generation of platelet additive solution for storage at 22 degrees C: development and current experience. Transfus Med Rev 2006;20:158-64.
16 HOW SUPPLIED/STORAGE AND HANDLING
Isoplate™ Solution Platelet Additive Solution [PAS-F] is supplied sterile and nonpyrogenic In EXCEL® Containers. The 500 mL containers are packaged 24 per case.
Storage
Store at 20°C to 25°C (68°F to 77°F), excursions permitted between 15°C and 30°C (between 59°F and 86°F). [See USP Controlled Room Temperature.]
Rx only
Issued: April 2013
EXCEL is a registered trademark of B. Braun Medical Inc.
Trima Accel is a registered trademark and Isoplate is a trademark of Terumo BCT Inc.
Manufactured for:
Terumo BCT, Inc
Lakewood, CO 80215 USA
Made in USA
Y36-002-833 LD-431-1
PRINCIPAL DISPLAY PANEL - 500 mL Container Label
Isoplate™ Solution
Platelet Additive Solution [PAS-F]
REF L7771
NDC 14537-408-50
500 mL
EXCEL
®
CONTAINER
Electrolytes (mEq/liter):
Na+ 141
K+ 5
Mg++ 3
Cl– 98
Acetate 27
Gluconate 23
Phosphate
(HPO=
4) 1
(0.5 mmole P/L)
Each 100 mL contains: Sodium Chloride USP 0.53 g;
Sodium Gluconate USP 0.5 g; Sodium Acetate•3H2O USP 0.37 g;
Potassium Chloride USP 0.037 g;Magnesium Chloride•6H2O USP 0.03 g;
Dibasic Sodium Phosphate•7H2O USP 0.012 g; Monobasic Potassium
Phosphate NF 0.00082 g; Water for Injection USP qs
pH may be adjusted with Glacial Acetic Acid USP or Sodium
Hydroxide NF
pH: 7.4 (7.0-7.8)
Calc. Osmolarity: 295 mOsmol/liter
Sterile, nonpyrogenic. Single use container.
Use only if solution is clear and container and seals are intact.
Warnings: Not for direct intravenous infusion.
Recommended Storage: Store at 20°C to 25°C (68°F to 77°F),
excursions permitted between 15°C and 30°C (between 59°F and 86°F).
[See USP Controlled Room Temperature.] See Package Insert.
Not made with natural rubber latex, PVC, or DEHP.
Rx only
EXCEL is a registered trademark of B. Braun Medical Inc.
Isoplate is a trademark of Terumo BCT Inc.
TERUMOBCT
Manufactured for:
Terumo BCT, Inc
Lakewood, CO 80215 USA
Made in USA
Y94-003-185
LD-430-1
Do not remove overwrap until ready for use. After removing the
overwrap, check for minute leaks by squeezing container firmly.
If leaks are found, discard solution as sterility may be impaired.

IsoplateSODIUM CHLORIDE, SODIUM GLUCONATE, SODIUM ACETATE, POTASSIUM CHLORIDE, MAGNESIUM CHLORIDE, SODIUM PHOSPHATE, DIBASIC, and POTASSIUM PHOSPHATE, MONOBASIC SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||