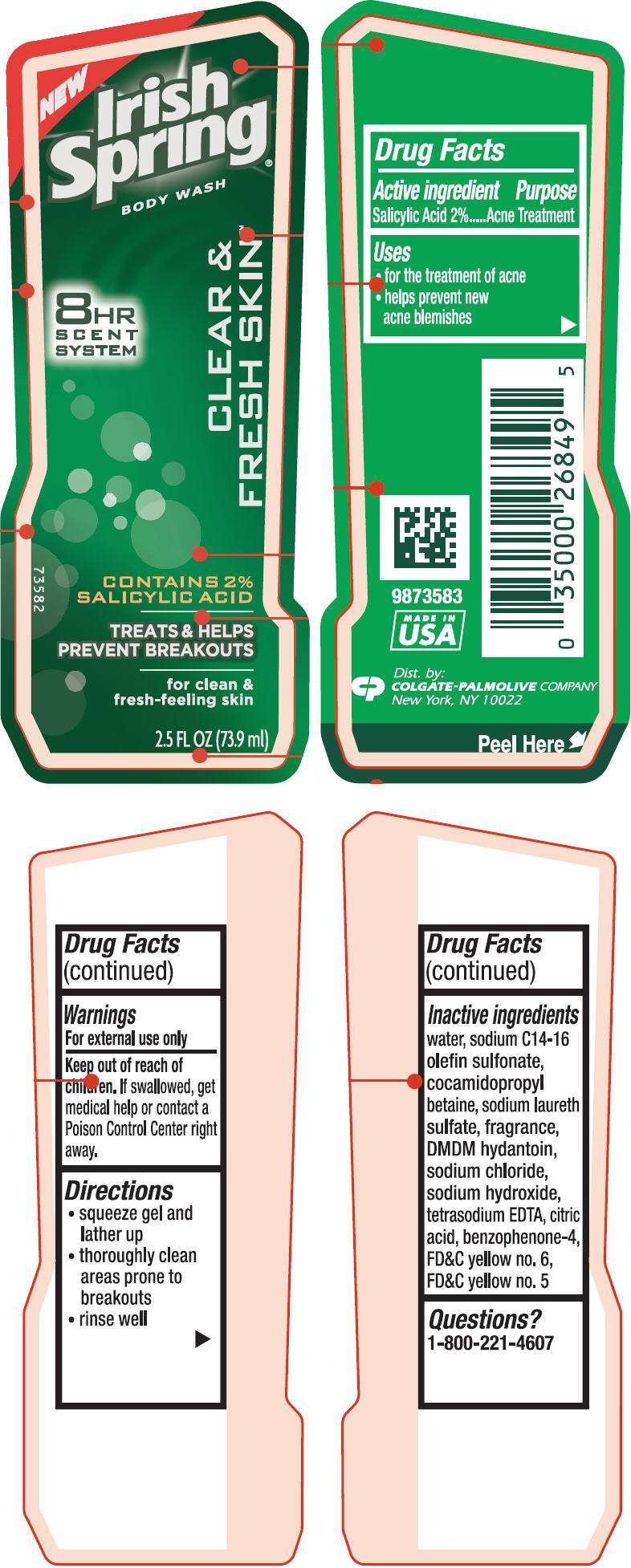
Irish Spring
Irish Spring Body Wash Clear & Fresh Skin™
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient
- Purpose
- Irish Spring Uses
- Warnings
- Directions
- Inactive ingredients
- Questions?
- PRINCIPAL DISPLAY PANEL - 73.9 ml Bottle Label
FULL PRESCRIBING INFORMATION
Drug Facts
Active ingredient
Salicylic Acid 2%
Purpose
Acne Treatment
Irish Spring Uses
- for the treatment of acne
- helps prevent new acne blemishes
Warnings
For external use only
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- squeeze gel and lather up
- thoroughly clean areas prone to breakouts
- rinse well
Inactive ingredients
water, sodium C14-16 olefin sulfonate, cocamidopropyl betaine, sodium laureth sulfate, fragrance, DMDM hydantoin, sodium chloride, sodium hydroxide, tetrasodium EDTA, citric acid, benzophenone-4, FD&C yellow no. 6, FD&C yellow no. 5
Questions?
1-800-221-4607
Dist. by:
COLGATE-PALMOLIVE
COMPANY
New York, NY 10022
PRINCIPAL DISPLAY PANEL - 73.9 ml Bottle Label
NEW
Irish
Spring
®
BODY WASH
8HR
SCENT
SYSTEM
CLEAR &
FRESH SKIN™
CONTAINS 2%
SALICYLIC ACID
TREATS & HELPS
PREVENT BREAKOUTS
for clean &
fresh-feeling skin
2.5 FL OZ (73.9 ml)
73582
Irish SpringSalicylic acid LIQUID
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||