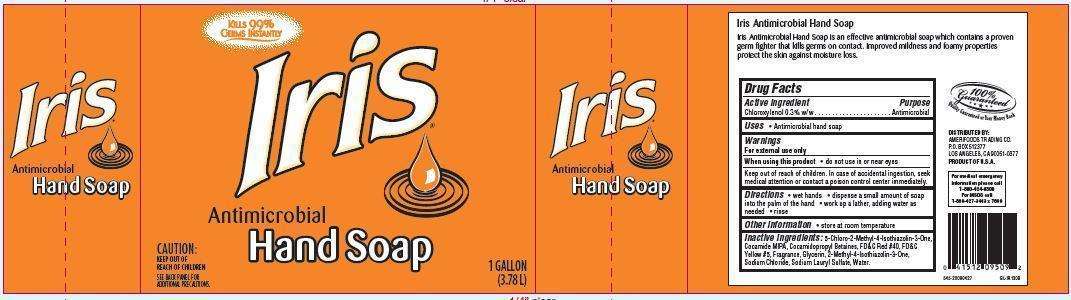
Iris Antimicrobial Hand
Iris Antimicrobial Hand Soap
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient
- Purpose
- Iris Antimicrobial Hand Uses
- Warnings
- Directions
- Iris Antimicrobial Hand Other information
- Inactive ingredients:
- Package Label
FULL PRESCRIBING INFORMATION
Active ingredient
Chloroxylenol 0.3% w/w
Purpose
Antimicrobial
Iris Antimicrobial Hand Uses
- Antimicrobial hand soap
Warnings
For external use only
When using this product
- do not use in or near eyes
Keep out of reach of children. In case of accidental ingestion, seek medical
attention or contact a poison control center immediately.
Directions
• wet hands • dispense a small amount of soap into the palm of
the hand • work up a lather, adding water as needed • rinse
Iris Antimicrobial Hand Other information
• store at room temperature
Inactive ingredients:
5-Chloro-2-Methyl-4-Isothiazolin-3-One,
Cocamide MIPA, Cocamidopropyl Betaines, FD&C Red #40, FD&C Yellow #5,
Fragrance, Glycerin, 2-Methyl-4-Isothiazolin-3-One, Sodium Chloride,
Sodium Lauryl Sulfate, Water.
Package Label
Kills 99%
Germs Instantly
Iris
Antimicrobial Hand Soap
CAUTION: KEEP OUT OF REACH OF CHILDREN.
SEE BACK PANEL FOR ADDITIONAL PRECAUTIONS.
1 GALLON
(3.78 L)
Iris Antimicrobial Hand Soap is an effective antimicrobial soap which contains a proven
germ fighter that kills germs on contact. Improved mildness and foamy properties
protect the skin against moisture loss.
100% Guaranteed
Quality Guaranteed or Your Money Back
DISTRIBUTED BY:
AMERIFOODS TRADING CO.
P.O. BOX 512377
LOS ANGELES, CA 90051-0377
PRODUCT OF U.S.A.
For medical emergency
information please call
1-800-424-9300
For MSDS call
1-800-427-3443 x 7600
Iris Antimicrobial HandCHLOROXYLENOL SOAP
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||