Irbesartan
Irbesartan Tablets, USP
FULL PRESCRIBING INFORMATION: CONTENTS*
- WARNING: FETAL TOXICITY
- IRBESARTAN DESCRIPTION
- CLINICAL PHARMACOLOGY
- IRBESARTAN INDICATIONS AND USAGE
- IRBESARTAN CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- IRBESARTAN ADVERSE REACTIONS
- OVERDOSAGE
- IRBESARTAN DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 75 mg (90 Tablet Bottle)
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 150 mg (90 Tablet Bottle)
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 150 mg Blister Carton (10 x 10 Unit-dose)
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 300 mg (90 Tablet Bottle)
FULL PRESCRIBING INFORMATION
WARNING: FETAL TOXICITY
- When pregnancy is detected, discontinue irbesartan as soon as possible.
- Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus. See WARNINGS: Fetal Toxicity.
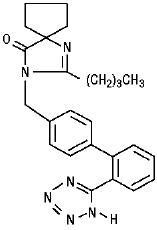
IRBESARTAN DESCRIPTION
1
poH
25286
CLINICAL PHARMACOLOGY
Mechanism of Action
12
1 12
1
Pharmacokinetics
Metabolism and Elimination
14
14
In vitro
Distribution
1
Special Populations
max
max
WARNINGS: Hypotension in Volume- or Salt-Depleted Patients DOSAGE AND ADMINISTRATION
PRECAUTIONS: Drug Interactions
Pharmacodynamics
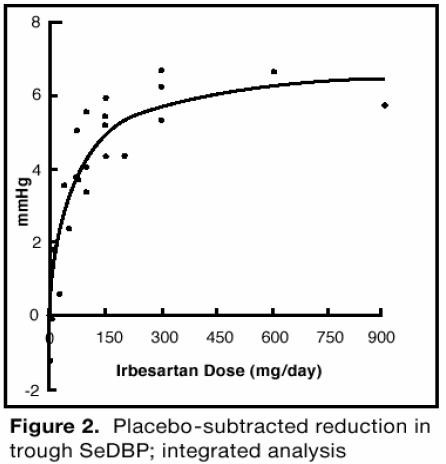
Clinical Studies

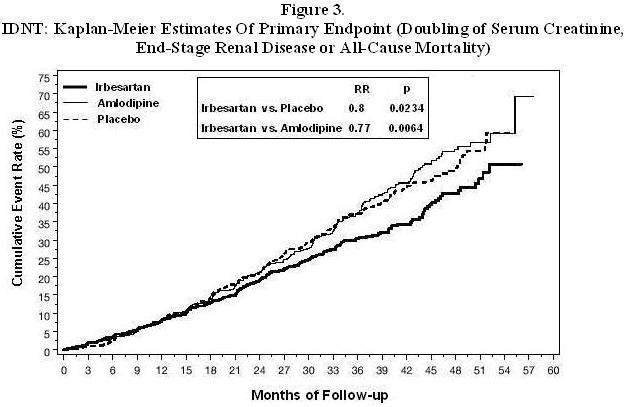
| Irbesartan N=579 (%) |
Comparison With Placebo | Comparison With Amlodipine | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Placebo N=569 (%) |
Hazard Ratio |
95% CI | Amlodipine N=567 (%) |
Hazard Ratio |
95% CI | ||||
| Primary Composite Endpoint |
32.6 |
39 |
0.8 |
0.66-0.97 (p=0.0234) |
41.1 |
0.77 |
0.63-0.93 |
||
| Breakdown of first occurring event contributing to primary endpoint |
|||||||||
| 2x creatinine |
14.2 |
19.5 |
--- |
--- |
22.8 |
--- |
--- |
||
| ESRD |
7.4 |
8.3 |
--- |
--- |
8.8 |
--- |
--- |
||
| Death |
11.1 |
11.2 |
--- |
--- |
9.5 |
--- |
--- |
||
| Incidence of total events over entire period of follow-up |
|||||||||
| 2x creatinine |
16.9 |
23.7 |
0.67 |
0.52-0.87 |
25.4 |
0.63 |
0.49-0.81 |
||
| ESRD |
14.2 |
17.8 |
0.77 |
0.57-1.03 |
18.3 |
0.77 |
0.57-1.03 |
||
| Death |
15 |
16.3 |
0.92 |
0.69-1.23 |
14.6 |
1.04 |
0.77-1.4 |
||
| Baseline Factors |
Irbesartan N=579 (%) |
Comparison With Placebo | ||
|---|---|---|---|---|
| Placebo N=569 (%) |
Hazard Ratio |
95% Cl | ||
| Gender |
||||
| Male |
27.5 |
36.7 |
0.68 |
0.53-0.88 |
| Female |
42.3 |
44.6 |
0.98 |
0.72-1.34 |
| Race |
||||
| White |
29.5 |
37.3 |
0.75 |
0.6-0.95 |
| Non-White |
42.6 |
43.5 |
0.95 |
0.67-1.34 |
| Age (years) |
||||
| < 65 |
31.8 |
39.9 |
0.77 |
0.62-0.97 |
| ≥ 65 |
35.1 |
36.8 |
0.88 |
0.61-1.29 |
IRBESARTAN INDICATIONS AND USAGE
Hypertension
Nephropathy in Type 2 Diabetic Patients
CLINICAL PHARMACOLOGY: Clinical Studies
IRBESARTAN CONTRAINDICATIONS
PRECAUTIONS, Drug Interactions
WARNINGS
Fetal Toxicity
in utero PRECAUTIONS: Pediatric Use
Hypotension in Volume- or Salt-Depleted Patients
DOSAGE AND ADMINISTRATION
PRECAUTIONS
Impaired Renal Function
Information for Patients
Drug Interactions
In vitro in vitro
Non-Steroidal Anti-Inflammatory Agents Including Selective Cyclooxygenase-2 Inhibitors (COX-2 Inhibitors)
Dual Blockade of the Renin-Angiotensin System (RAS)
Carcinogenesis, Mutagenesis, Impairment of Fertility
0-24 hour
in vitroin vitroin vivo
0-24 hour
Pregnancy
Pregnancy Category D
WARNINGS: Fetal Toxicity
Nursing Mothers
Pediatric Use
Neonates with a history of in utero exposure to irbesartan
Geriatric Use
CLINICAL PHARMACOLOGY: Pharmacokinetics Special Populations Clinical Studies
IRBESARTAN ADVERSE REACTIONS
Hypertension
Body as a Whole:
Cardiovascular:
Dermatologic:
Endocrine/Metabolic/Electrolyte Imbalances:
Gastrointestinal:
Musculoskeletal/Connective Tissue:
Nervous System:
Renal/Genitourinary:
Respiratory:
Special Senses:
Nephropathy in Type 2 Diabetic Patients
Postmarketing Experience
Laboratory Test Findings
Creatinine, Blood Urea Nitrogen: PRECAUTIONS: Impaired Renal Function
Hematologic: 3
Hyperkalemia:
OVERDOSAGE
Physicians’ Desk Reference
2
IRBESARTAN DOSAGE AND ADMINISTRATION
Hypertension
CLINICAL PHARMACOLOGY: Clinical Studies
Nephropathy in Type 2 Diabetic Patients
CLINICAL PHARMACOLOGY: Clinical Studies
Volume- and Salt-Depleted Patients
WARNINGS: Hypotension in Volume- or Salt-Depleted Patients
HOW SUPPLIED
Irbesartan Tablets USP, 75 mg
Irbesartan Tablets USP, 150 mg
Irbesartan Tablets USP, 300 mg
Storage
Store at
Aurobindo Pharma USA, Inc.
Aurobindo Pharma Limited
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 75 mg (90 Tablet Bottle)
NDC 65862-637-90
Irbesartan Tablets, USP
75 mg
Rx only 90 Tablets
AUROBINDO

PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 150 mg (90 Tablet Bottle)
NDC 65862-638-90
Irbesartan Tablets, USP
150 mg
Rx only 90 Tablets
AUROBINDO

PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 150 mg Blister Carton (10 x 10 Unit-dose)
NDC 65862-638-78
Irbesartan Tablets, USP
150 mg
Rx only 100 Tablets ( 10 x 10 Unit-dose)
AUROBINDO
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 300 mg (90 Tablet Bottle)
NDC 65862-639-90
Irbesartan Tablets, USP
300 mg
Rx only 90 Tablets
AUROBINDO

IrbesartanIrbesartan TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
IrbesartanIrbesartan TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
IrbesartanIrbesartan TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!