IOPE RETIGEN MOISTURE FOUNDATION
IOPE Retigen Moisture Foundation
FULL PRESCRIBING INFORMATION: CONTENTS*
- ACTIVE INGREDIENTS
- PURPOSE
- USES
- WARNINGS
- DIRECTIONS
- IOPE RETIGEN MOISTURE FOUNDATION Other information
- INACTIVE INGREDIENTS
- PRINCIPAL DISPLAY PANEL - 35 ml Bottle Carton - No. 17
- PRINCIPAL DISPLAY PANEL - 35 ml Bottle Carton - No. 21
- PRINCIPAL DISPLAY PANEL - 35 ml Bottle Carton - No. 23
FULL PRESCRIBING INFORMATION
Drug Facts
ACTIVE INGREDIENTS
| ETHYLHEXYL METHOXYCINNAMATE | 5% |
| TITANIUM DIOXIDE | 2.49% |
| ZINC OXIDE | 0.98% |
PURPOSE
Sunscreen
USES
Helps prevent sunburn. If used as directed with other sun protection measures (see Directions ), decreases the risk of skin cancer and early skin aging caused by the sun.
WARNINGS
For external use only.
Do not use on damaged or broken skin.
When using this product keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if rash occurs.
Keep out of reach of children. If product is swallowed, get medical help or contact a Poison Control Center right away.
DIRECTIONS
Apply liberally 15 minutes before sun exposure. Reapply at least every 2 hours. Use a water-resistant sunscreen if swimming or sweating. Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: Limit time in the sun, especially from 10 a.m. - 2 p.m. Wear long-sleeved shirts, pants, hats, and sunglasses. Children under 6 months of age: Ask a doctor.
IOPE RETIGEN MOISTURE FOUNDATION Other information
Protect the product in this container from excessive heat and direct sun.
INACTIVE INGREDIENTS
WATER, CYCLOPENTASILOXANE, TITANIUM DIOXIDE (CI 77891), CYCLOHEXASILOXANE, BUTYLENE GLYCOL, CETYL PEG/PPG-10/1 DIMETHICONE, METHYL TRIMETHICONE, DIMETHICONE, SODIUM CHLORIDE, IRON OXIDES (CI 77492), OZOKERITE, PHENYL TRIMETHICONE, SORBITAN ISOSTEARATE, DISTEARDIMONIUM HECTORITE, DICAPRYLYL CARBONATE, POLYMETHYL METHACRYLATE, SQUALANE, POLYSILICONE-11, VINYL DIMETHICONE/METHICONE SILSESQUIOXANE CROSSPOLYMER, ALUMINUM HYDROXIDE, ETHYLHEXYLGLYCERIN, BEESWAX, COPERNICIA CERIFERA (CARNAUBA) WAX, HYDROGENATED CASTOR OIL ISOSTEARATE, RICINUS COMMUNIS (CASTOR) SEED OIL, STEARIC ACID, IRON OXIDES (CI 77491), TRIETHOXYCAPRYLYLSILANE, CAPRYLYL GLYCOL, FRAGRANCE, CALCIUM STEARATE, SORBITAN SESQUIOLEATE, ACRYLATES/AMMONIUM METHACRYLATE COPOLYMER, IRON OXIDES (CI 77499), DISODIUM EDTA, POLYACRYLIC ACID, CAMELLIA SINENSIS LEAF EXTRACT, HYDROLYZED EXTENSIN, BETA-CAROTENE, GLYCERIN, PROPANEDIOL, BETA-GLUCAN
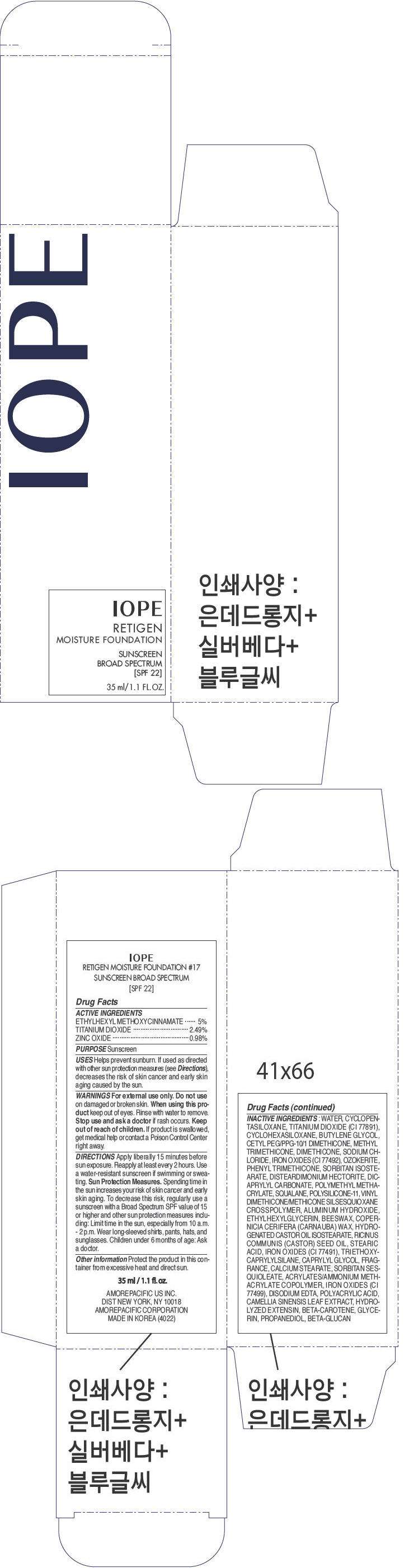
PRINCIPAL DISPLAY PANEL - 35 ml Bottle Carton - No. 17
IOPE
IOPE
RETIGEN
MOISTURE FOUNDATION
SUNSCREEN
BROAD SPECTRUM
[SPF 22]
35 ml/1.1 FL.OZ.
PRINCIPAL DISPLAY PANEL - 35 ml Bottle Carton - No. 21
IOPE
IOPE
RETIGEN
MOISTURE FOUNDATION
SUNSCREEN
BROAD SPECTRUM
[SPF 22]
35 ml/1.1 FL.OZ.

PRINCIPAL DISPLAY PANEL - 35 ml Bottle Carton - No. 23
IOPE
IOPE
RETIGEN
MOISTURE FOUNDATION
SUNSCREEN
BROAD SPECTRUM
[SPF 22]
35 ml/1.1 FL.OZ.

IOPE RETIGEN MOISTURE FOUNDATIONOCTINOXATE, TITANIUM DIOXIDE, and ZINC OXIDE LOTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
IOPE RETIGEN MOISTURE FOUNDATIONOCTINOXATE, TITANIUM DIOXIDE, and ZINC OXIDE LOTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
IOPE RETIGEN MOISTURE FOUNDATIONOCTINOXATE, TITANIUM DIOXIDE, and ZINC OXIDE LOTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||