IOPE MILD CLINIC SUN PROTECTOR
IOPE MILD CLINIC SUN PROTECTOR
FULL PRESCRIBING INFORMATION: CONTENTS*
- ACTIVE INGREDIENTS
- Purpose
- Use
- Warnings
- Directions
- INACTIVE INGREDIENTS
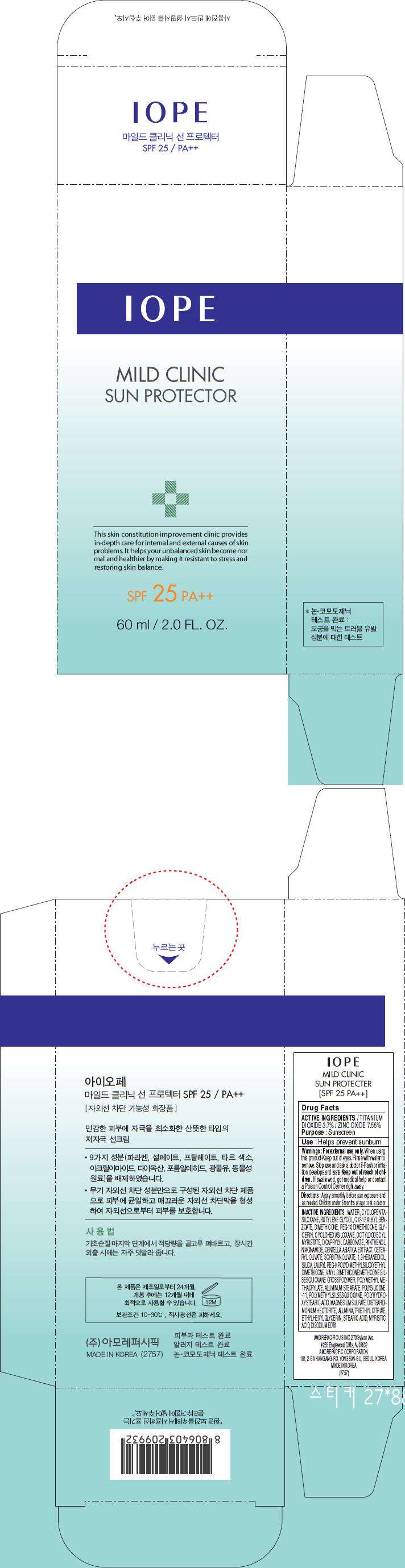
- PRINCIPAL DISPLAY PANEL - 60 ml Carton
FULL PRESCRIBING INFORMATION
Drug Facts
ACTIVE INGREDIENTS
TITANIUM DIOXIDE 3.7% / ZINC OXIDE 7.55%
Purpose
Sunscreen
Use
Helps prevent sunburn
Warnings
For external use only.
When using this product
Keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if
Rash or irritation develops and lasts
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Apply smoothly before sun exposure and as needed. Children under 6 months of age : ask a doctor
INACTIVE INGREDIENTS
WATER, CYCLOPENTASILOXANE, BUTYLENE GLYCOL, C12-15 ALKYL BENZOATE, DIMETHICONE, PEG-10 DIMETHICONE, GLYCERIN, CYCLOHEXASILOXANE, OCTYLDODECYL MYRISTATE, DICAPRYLYL CARBONATE, PANTHENOL, NIACINAMIDE, CENTELLA ASIATICA EXTRACT, CETEARYL OLIVATE, SORBITAN OLIVATE, 1,2-HEXANEDIOL, SILICA, LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE, VINYL DIMETHICONE/METHICONE SILSESQUIOXANE CROSSPOLYMER, POLYMETHYL METHACRYLATE, ALUMINUM STEARATE, POLYSILICONE-11, POLYMETHYLSILSESQUIOXANE, POLYHYDROXYSTEARIC ACID, MAGNESIUM SULFATE, DISTEARDIMONIUM HECTORITE, ALUMINA, TRIETHYL CITRATE, ETHYLHEXYLGLYCERIN, STEARIC ACID, MYRISTIC ACID, DISODIUM EDTA
PRINCIPAL DISPLAY PANEL - 60 ml Carton
IOPE
MILD CLINIC
SUN PROTECTOR
This skin constitution improvement clinic provides
in-depth care for internal and external causes of skin
problems. It helps your unbalanced skin become nor
mal and healthier by making it resistant to stress and
restoring skin balance.
SPF 25 PA++
60 ml / 2.0 FL. OZ.
IOPE MILD CLINIC SUN PROTECTORTITANIUM DIOXIDE and ZINC OXIDE CREAM
| ||||||||||||||||||||||||||||||||||||||||||||||||||||