IOPE Air Cushion
IOPEAir Cushion
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
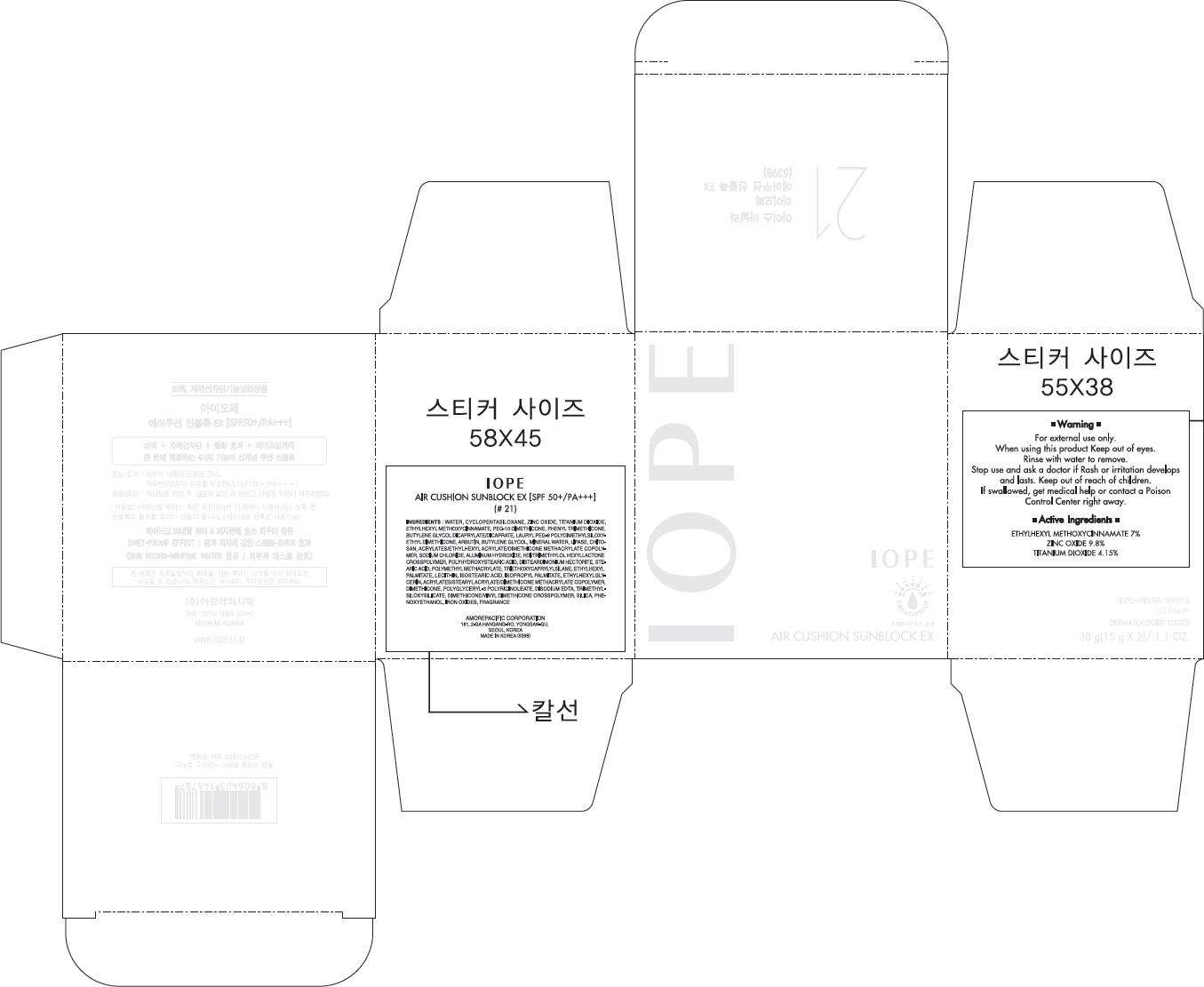
IOPE
AIR CUSHION SUNBLOCK EX [SPF 50+/PA+++]
(# 21)
INGREDIENTS
WATER, CYCLOPENTASILOXANE, ZINC OXIDE, TITANIUM DIOXIDE, ETHYLHEXYL METHOXYCINNAMATE, PEG-10 DIMETHICONE, PHENYL TRIMETHICONE, BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE, LAURYL PEG-9 POLYDIMETHYLSILOXY-ETHYL DIMETHICONE, ARBUTIN, BUTYLENE GLYCOL, MINERAL WATER, LIPASE, CHITOSAN, ACRYLATES/ETHYLHEXYL ACRYLATE/DIMETHICONE METHACRYLATE COPOLYMER, SODIUM CHLORIDE, ALUMINUM HYDROXIDE, HDI/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER, POLYHYDROXYSTEARIC ACID, DISTEARDIMONIUM HECTORITE, STEARIC ACID, POLYMETHYL METHACRYLATE, TRIETHOXYCAPRYLYLSILANE, ETHYLHEXYL PALMITATE, LECITHIN, ISOSTEARIC ACID, ISOPROPYL PALMITATE, ETHYLHEXYLGLYCERIN, ACRYLATES/STEARYL ACRYLATE/DIMETHICONE METHACRYLATE COPOLYMER, DIMETHICONE, POLYGLYCERYL-3 POLYRICINOLEATE, DISODIUM EDTA, TRIMETHYLSILOXYSILICATE, DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER, SILICA, PHENOXYETHANOL, IRON OXIDES, FRAGRANCE
Warning
For external use only.
When using this product Keep out of eyes.
Rinse with water to remove.
Stop use and ask a doctor if Rash or irritation develops and lasts.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Active Ingredients
ETHYLHEXYL METHOXYCINNAMATE 7%
ZINC OXIDE 9.8%
TITANIUM DIOXIDE 4.15%
PRINCIPAL DISPLAY PANEL - 30 g Carton
IOPE
IOPE
SWEAT-PROOF
AIR CUSHION SUNBLOCK EX
IOPE Air CushionOCTINOXATE, TITANIUM DIOXIDE, and ZINC OXIDE CREAM
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||