Instant Foam Hand Sanitizer
Dixie Packing & Seal Company
ABC Compounding Co., Inc.
Instant Foam Hand Sanitizer 6585 Drug Facts and Label
FULL PRESCRIBING INFORMATION: CONTENTS*
- Drug Facts Box OTC-Active Ingredient Section
- Drug Facts Box OTC-Purpose Section
- Drug Facts Box OTC-Indications & Usage Section
- Drug Facts Box OTC-Warnings Section
- Drug Facts Box OTC-When Using Section
- Drug Facts Box OTC-Stop Use Section
- Drug Facts Box OTC-Keep Out of Reach of Children Section
- Drug Facts Box OTC-Dosage & Administration Section
- Drug Facts Box OTC-Inactive Ingredient Section
- Instant Foam Hand Sanitizer 6585 50ml
FULL PRESCRIBING INFORMATION
Drug Facts Box OTC-Active Ingredient Section
Ethyl Alcohol 62%
Drug Facts Box OTC-Purpose Section
Antiseptic
Drug Facts Box OTC-Indications & Usage Section
for hand-washing to decrease bacteria on the skin, only when water is not available
Drug Facts Box OTC-Warnings Section
FLAMMABLE, keep away from fire and flames
For external use only
Drug Facts Box OTC-When Using Section
do not get into eyes
if contact occurs, rinse eyes thoroughly with water
Drug Facts Box OTC-Stop Use Section
irritation and redness develop
Drug Facts Box OTC-Keep Out of Reach of Children Section
if swallowed, get medical help or contact a Poison Control Center right away
Drug Facts Box OTC-Dosage & Administration Section
press pump twice to deliver two squirts (about a quarter size) of foaming product onto the palm of your hand
rub hands together and allow to dry without wiping
Drug Facts Box OTC-Inactive Ingredient Section
water, DEA-C8-18 perfluoroalkylethyl phosphate, propylene glycol, fragrance
Instant Foam Hand Sanitizer 6585 50ml
6585Z1Q90000.jpg Instant Foam Hand Sanitizer 50ml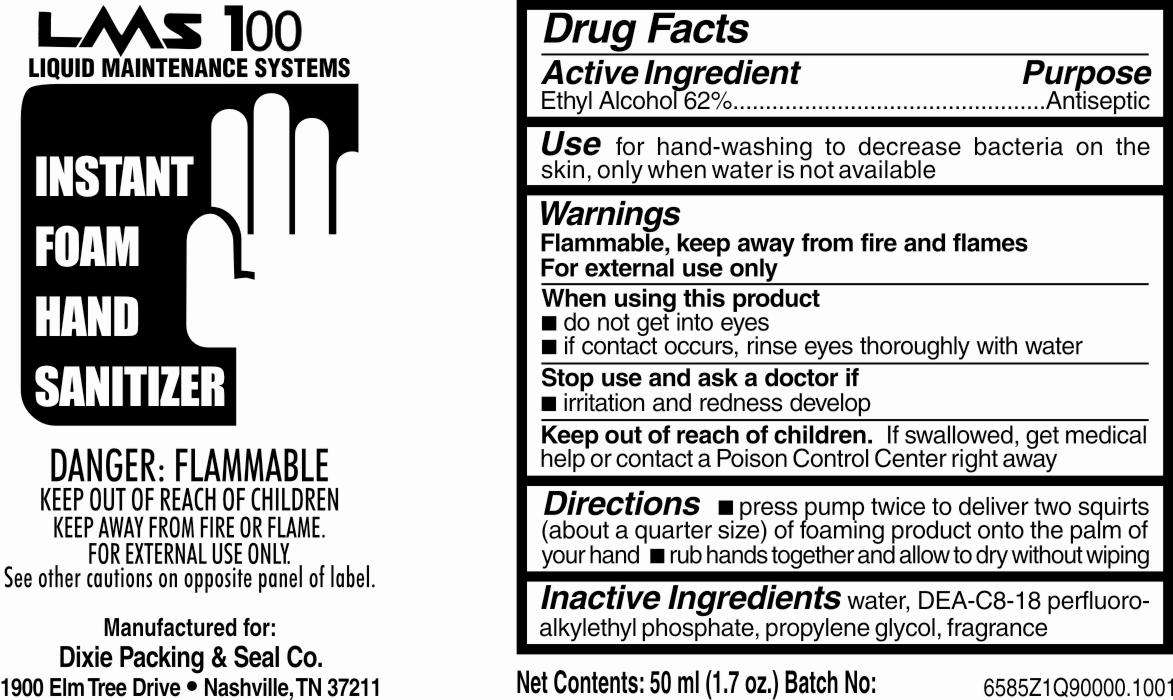
Instant Foam Hand SanitizerALCOHOL LIQUID
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||