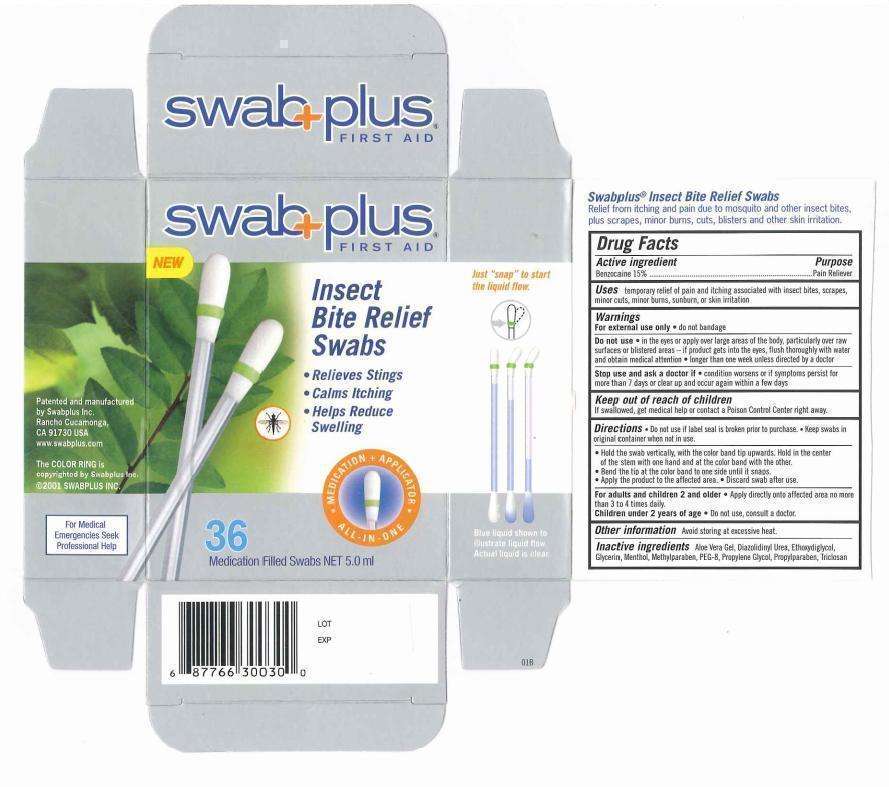
Insect Bite Relief
Insect Bite Relief Swab
FULL PRESCRIBING INFORMATION
Active ingredient
Drug Fact
Active ingredient
Benzocaine 15%
Purpose
Purpose: Benzocaine for Pain Relief
Use: temporary relief of pain and itching associated with insect bites, scrapes, minor cuts, minor burns, sunburn, or skin irritation.
Warnings
For external use only. do not bandage.
Do not use. in the eyes or apply over large areas of the body. particularly over raw surfaces or blistered area - if product gets into the eyes. flush throughly with water and obtain medical attention. longer than one week unless directed by doctor.
Stop use and ask a doctor if.
If swallowed, get medical help or contact a Poison Control Center right away.
Uses
Directions. Do not use if label seal is broken prior to purchase. Keep swabs in original container when not in use.
- Hold the swab vertically, with the color band tip upwards. hold in the center of the stem with one hand and at the color band with the other.
- Bend the tip at the color band to one side until it snaps.
- Apply the product to the affected area.
- Discard swab after use.
For adult and children 2 and older. Apply directly onto affected area no more than 3 to 4 times daily.
Children under 2 years of age. Do not use, consult a doctor.
Other information. Avoid storing at excessive heat.
Aloe Vera Gel. Diazolidinyly Urea, Glycerin, Menthol, Methylparaben, Peg-8, Propylene Glycol, Propylparaben.
Image of insect bite carton label
Insect Bite ReliefBenzocaine LIQUID
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||