INFUMORPH 200
West-ward Pharmaceutical Corp.
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
Morphine is the most important alkaloid of opium and is a phenanthrene derivative. It is available as the sulfate salt, having the following structural formula:
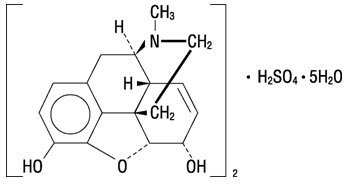
7,8-Didehydro-4,5-epoxy-17-methyl-(5α,6α)-morphinan-3,6-diol sulfate (2:1) (salt), pentahydrate
(C17H19NO3)2• H2SO4• 5H2O MW is 758.83
INFUMORPH is a sterile, nonpyrogenic, isobaric, high potency solution of morphine sulfate, free of antioxidants, preservatives or other potentially neurotoxic additives. INFUMORPH is intended for use in continuous microinfusion devices for intraspinal administration in the management of pain.
Each 20 mL ampul of INFUMORPH 200 contains morphine sulfate, USP 200 mg or 10 mg/mL and sodium chloride 8 mg/mL in Water for Injection, USP. Each 20 mL ampul of INFUMORPH 500 contains morphine sulfate, USP 500 mg or 25 mg/mL and sodium chloride 6.25 mg/mL in Water for Injection, USP. If needed, sodium hydroxide and/or sulfuric acid are added for pH adjustment to 4.5. Each 20 mL ampul of INFUMORPH is intended for single use only. Discard any unused portion. DO NOT HEAT-STERILIZE.
Morphine produces a wide spectrum of pharmacologic effects including analgesia, dysphoria, euphoria, somnolence, respiratory depression, diminished gastrointestinal motility and physical dependence. Opiate analgesia involves at least three anatomical areas of the central nervous system: the periaqueductal-periventricular gray matter, the ventromedial medulla and the spinal cord. A systemically administered opiate may produce analgesia by acting at any, all or some combination of these distinct regions. Morphine interacts predominantly with the µ-receptor. The µ-binding sites of opioids are very discretely distributed in the human brain, with high densities of sites found in the posterior amygdala, hypothalamus, thalamus, nucleus caudatus, putamen and certain cortical areas. They are also found on the terminal axons of primary afferents within laminae I and II (substantia gelatinosa) of the spinal cord and in the spinal nucleus of the trigeminal nerve.
Morphine has an apparent volume of distribution ranging from 1.0 to 4.7 L/kg after intravenous dosage. Protein binding is low, about 36%, and muscle tissue binding is reported as 54%. A blood-brain barrier exists, and when morphine is introduced outside of the CNS (e.g., intravenously), plasma concentrations of morphine remain higher than the corresponding CSF morphine levels. Conversely, when morphine is injected into the intrathecal space, it diffuses out into the systemic circulation slowly, accounting for the long duration of action of morphine administered by this route.
Morphine has a total plasma clearance which ranges from 0.9 to 1.2 L/kg/h (liters/kilogram/hour) in postoperative patients, but shows considerable interindividual variation. The major pathway of clearance is hepatic glucuronidation to morphine‑3‑glucuronide, which is pharmacologically inactive. The major excretion path of the conjugate is through the kidneys, with about 10% in the feces. Morphine is also eliminated by the kidneys, 2 to 12% being excreted unchanged in the urine. Terminal half-life is commonly reported to vary from 1.5 to 4.5 hours, although the longer half‑lives were obtained when morphine levels were monitored over protracted periods with very sensitive radioimmunoassay methods. The accepted elimination half-life in normal subjects is 1.5 to 2 hours.
“Selective” blockade of pain sensation is possible by neuraxial application of morphine. In addition, duration of analgesia may be much longer by this route compared to systemic administration. However, CNS effects, associated with systemic administration, are still seen. These include respiratory depression, sedation, nausea and vomiting, pruritus and urinary retention. In particular, both early and late respiratory depression (up to 24 hours post dosing) have been reported following neuraxial administration. Circulation of the spinal fluid may also result in high concentrations of morphine reaching the brain stem directly.
The incidence of unwanted CNS effects, including delayed respiratory depression, associated with neuraxial application of morphine, is related to the circulatory dynamics of the epidural venous plexus and the spinal fluid. The lipid solubility and degree of ionization of morphine plays an important part in both the onset and duration of analgesia and the CNS effects. Morphine has a pKa 7.9, with an octanol/water partition coefficient of 1.42 at pH 7.4. At this pH, the tertiary amino group in each of the opioids is mostly ionized, making the molecule water soluble. Morphine, with additional hydroxyl groups on the molecule, is significantly more water soluble than any other opioid in clinical use.
Morphine, injected into the epidural space, is rapidly absorbed into the general circulation. Absorption is so rapid that the plasma concentration-time profiles closely resemble those obtained after intravenous or intramuscular administration. Peak plasma concentrations averaging 33-40 ng/mL (range 5-62 ng/mL) are achieved within 10 to 15 minutes after administration of 3 mg of morphine. Plasma concentrations decline in a multiexponential fashion. The terminal half-life is reported to range from 39 to 249 minutes (mean of 90 ± 34.3 min) and, though somewhat shorter, is similar in magnitude as values reported after intravenous and intramuscular administration (1.5-4.5 h). CSF concentrations of morphine, after epidural doses of 2 to 6 mg in postoperative patients, have been reported to be 50 to 250 times higher than corresponding plasma concentrations. The CSF levels of morphine exceed those in plasma after only 15 minutes and are detectable for as long as 20 hours after the injection of 2 mg of epidural morphine. Approximately 4% of the dose injected epidurally reaches the CSF. This corresponds to the relative minimum effective epidural and intrathecal doses of 5 mg and 0.25 mg, respectively. The disposition of morphine in the CSF follows a biphasic pattern, with an early half-life of 1.5 h and a late phase half-life of about 6 h. Morphine crosses the dura slowly, with an absorption half-life across the dura averaging 22 minutes. Maximum CSF concentrations are seen 60-90 minutes after injection. Minimum effective CSF concentrations for postoperative analgesia average 150 ng/mL (range <1‑380 ng/mL).
The intrathecal route of administration circumvents meningeal diffusion barriers and, therefore, lower doses of morphine produce comparable analgesia to that induced by the epidural route. After intrathecal bolus injection of morphine, there is a rapid initial distribution phase lasting 15-30 minutes and a half-life in the CSF of 42-136 min (mean 90 ± 16 min). Derived from limited data, it appears that the disposition of morphine in the CSF, from 15 minutes postintrathecal administration to the end of a six-hour observation period, represents a combination of the distribution and elimination phases. Morphine concentrations in the CSF averaged 332 ± 137 ng/mL at 6 hours, following a bolus dose of 0.3 mg of morphine. The apparent volume of distribution of morphine in the intrathecal space is about 22 ± 8 mL.
Time-to-peak plasma concentrations, however, is similar (5-10 min) after either epidural or intrathecal bolus administration of morphine. Maximum plasma morphine concentrations after 0.3 mg intrathecal morphine have been reported from <1 to 7.8 ng/mL. The minimum analgesic morphine plasma concentration during Patient‑Controlled Analgesia (PCA) has been reported as 20-40 ng/mL, suggesting that any analgesic contribution from systemic redistribution would be minimal after the first 30-60 minutes with epidural administration and virtually absent with intrathecal administration of morphine.
INFUMORPH (Preservative-free Morphine Sulfate Sterile Solution) is indicated only for intrathecal or epidural infusion in the treatment of intractable chronic pain. It was developed for use in continuous microinfusion devices and may require dilution before use as dictated by the characteristics of the device and the dosage requirements of the individual patient.
|
INFUMORPH IS NOT RECOMMENDED FOR SINGLE-DOSE INTRAVENOUS, INTRAMUSCULAR OR SUBCUTANEOUS ADMINISTRATION DUE TO THE VERY LARGE AMOUNT OF MORPHINE IN THE AMPUL AND THE ASSOCIATED RISK OF OVERDOSAGE. |
The only absolute contraindication to the use of INFUMORPH is known allergy to morphine. Contraindications to the use of neuraxial analgesia include: the presence of infection at the injection microinfusion site, concomitant anticoagulant therapy, uncontrolled bleeding diathesis and the presence of any other concomitant therapy or medical condition which would render epidural or intrathecal administration of medication especially hazardous.
Morphine sulfate may be habit forming. Overdoses may cause respiratory depression, coma, and death.
THIS PRODUCT WAS DEVELOPED FOR USE (AFTER APPROPRIATE DILUTION, IF NECESSARY) IN CONTINUOUS MICROINFUSION DEVICES FOR INTRATHECAL OR EPIDURAL INFUSION OF NARCOTICS TO CONTROL SEVERE CANCER PAIN. CHRONIC NEURAXIAL OPIOID ANALGESIA IS APPROPRIATE ONLY WHEN LESS INVASIVE MEANS OF CONTROLLING PAIN HAVE FAILED AND SHOULD ONLY BE UNDERTAKEN BY THOSE WHO ARE EXPERIENCED IN APPLYING THIS TREATMENT IN A SETTING WHERE ITS COMPLICATIONS CAN BE ADEQUATELY MANAGED.
|
BECAUSE OF THE RISK OF SEVERE ADVERSE EFFECTS, PATIENTS MUST BE OBSERVED IN A FULLY EQUIPPED AND STAFFED ENVIRONMENT FOR AT LEAST 24 HOURS AFTER THE INITIAL (SINGLE) TEST DOSE AND, AS APPROPRIATE, FOR THE FIRST SEVERAL DAYS AFTER CATHETER IMPLANTATION. |
THE FACILITY MUST BE EQUIPPED TO RESUSCITATE PATIENTS WITH SEVERE OPIATE OVERDOSAGE, AND THE PERSONNEL MUST BE FAMILIAR WITH THE USE AND LIMITATIONS OF SPECIFIC NARCOTIC ANTAGONISTS (NALOXONE, NALTREXONE) IN SUCH CASES.
RESERVOIR FILLING MUST BE PERFORMED BY FULLY TRAINED AND QUALIFIED PERSONNEL, FOLLOWING THE DIRECTIONS PROVIDED BY THE DEVICE MANUFACTURER. CARE SHOULD BE TAKEN IN SELECTING THE PROPER REFILL FREQUENCY TO PREVENT DEPLETION OF THE RESERVOIR, WHICH WOULD RESULT IN EXACERBATION OF SEVERE PAIN AND/OR REFLUX OF CSF INTO SOME DEVICES. STRICT ASEPTIC TECHNIQUE IN FILLING IS REQUIRED TO AVOID BACTERIALCONTAMINATION AND SERIOUS INFECTION. EXTREME CARE MUST BE TAKEN TO ENSURE THAT THE NEEDLE IS PROPERLY IN THE FILLING PORT OF THE DEVICE BEFORE ATTEMPTING TO REFILL THE RESERVOIR. INJECTING THE SOLUTION INTO THE TISSUE AROUND THE DEVICE OR (IN THE CASE OF DEVICES THAT HAVE MORE THAN ONE PORT) ATTEMPTING TO INJECT THE REFILL DOSE INTO THE DIRECT INJECTION PORT WILL RESULT IN A LARGE, CLINICALLY SIGNIFICANT, OVERDOSAGE TO THE PATIENT.
A PERIOD OF OBSERVATION APPROPRIATE TO THE CLINICAL SITUATION SHOULD FOLLOW EACH REFILL OR MANIPULATION OF THE DRUG RESERVOIR. BEFORE DISCHARGE, THE PATIENT AND ATTENDANT(S) SHOULD RECEIVE INSTRUCTION IN THE PROPER HOME CARE OF THE DEVICE AND INSERTION SITE AND IN THE RECOGNITION AND PRACTICAL TREATMENT OF AN OVERDOSE OF NEURAXIAL MORPHINE.
INFLAMMATORY MASSES SUCH AS GRANULOMAS, SOME OF WHICH HAVE RESULTED IN SERIOUS NEUROLOGIC IMPAIRMENT INCLUDING PARALYSIS, HAVE BEEN REPORTED TO OCCUR IN PATIENTS RECEIVING CONTINUOUS INFUSION OF OPIOID ANALGESICS INCLUDING INFUMORPH VIA INDWELLING INTRATHECAL CATHETER. PATIENTS RECEIVING CONTINUOUS INFUSION OF INFUMORPH VIA INDWELLING INTRATHECAL CATHETER SHOULD BE CAREFULLY MONITORED FOR NEW NEUROLOGIC SIGNS OR SYMPTOMS. FURTHER ASSESSMENT OR INTERVENTION SHOULD BE BASED ON THE CLINICAL CONDITION OF THE INDIVIDUAL PATIENT.
PATIENTS SOMETIMES MANIFEST UNUSUAL ACCELERATION OF NEURAXIAL MORPHINE REQUIREMENTS, WHICH MAY CAUSE CONCERN REGARDING SYSTEMIC ABSORPTION AND THE HAZARDS OF LARGE DOSES; THESE PATIENTS MAY BENEFIT FROM HOSPITALIZATION AND DETOXIFICATION. TWO CASES OF MYOCLONIC-LIKE SPASM OF THE LOWER EXTREMITIES HAVE BEEN REPORTED IN PATIENTS RECEIVING MORE THAN 20 MG/DAY OF INTRATHECAL MORPHINE. AFTER DETOXIFICATION, IT MIGHT BE POSSIBLE TO RESUME TREATMENT AT LOWER DOSES, AND SOME PATIENTS HAVE BEEN SUCCESSFULLY CHANGED FROM CONTINUOUS EPIDURAL MORPHINE TO CONTINUOUS INTRATHECAL MORPHINE. REPEAT DETOXIFICATION MAY BE INDICATED AT A LATER DATE. THE UPPER DAILY DOSAGE LIMIT FOR EACH PATIENT DURING CONTINUING TREATMENT MUST BE INDIVIDUALIZED.
Control of pain by neuraxial opiate delivery, using a continuous microinfusion device, is always accompanied by considerable risk to the patients and requires a high level of skill to be successfully accomplished. The task of treating these patients must be undertaken by experienced clinical teams, well-versed in patient selection, evolving technology and emerging standards of care. For reasons of safety, it is recommended that administration of INFUMORPH 200 and 500 (10 and 25 mg/mL, respectively) by the intrathecal route be limited to the lumbar area.
INFUMORPH (Preservative-free Morphine Sulfate Sterile Solution) should be used with extreme caution in patients with head injury or increased intracranial pressure. Pupillary changes (miosis) from morphine may obscure the existence, extent and course of intracranial pathology. High doses of neuraxial morphine may produce myoclonic events (see WARNINGS and ADVERSE REACTIONS ). Clinicians should maintain a high index of suspicion for adverse drug reactions when evaluating altered mental status or movement abnormalities in patients receiving this modality of treatment.
Care is urged in using this drug in patients who have a decreased respiratory reserve (e.g., emphysema, severe obesity, kyphoscoliosis or paralysis of the phrenic nerve). INFUMORPH should not be given in cases of chronic asthma, upper airway obstruction or in any other chronic pulmonary disorder without due consideration of the known risk of acute respiratory failure following morphine administration in such patients.
The elimination half-life of morphine may be prolonged in patients with reduced metabolic rates and with hepatic and/or renal dysfunction. Hence, care should be exercised in administering INFUMORPH epidurally to patients with these conditions, since high blood morphine levels, due to reduced clearance, may take several days to develop.
As significant morphine is released into the systemic circulation from neuraxial administration, the ensuing smooth muscle hypertonicity may result in biliary colic.
Initiation of neuraxial opiate analgesia is frequently associated with disturbances of micturition, especially in males with prostatic enlargement. Early recognition of difficulty in urination and prompt intervention in cases of urinary retention is indicated.
Patients with reduced circulating blood volume, impaired myocardial function or on sympatholytic drugs should be monitored for the possible occurrence of orthostatic hypotension, a frequent complication in single-dose neuraxial morphine analgesia.
The depressant effects of morphine are potentiated by the presence of other CNS depressants such as alcohol, sedatives, antihistaminics or psychotropic drugs. Use of neuroleptics in conjunction with neuraxial morphine may increase the risk of respiratory depression.
Morphine is without known carcinogenic or mutagenic effects and is not known to impair fertility at non-narcotic doses in animals, but studies of the carcinogenic and mutagenic potential or the effect on fertility of INFUMORPH (Preservative-free Morphine Sulfate Sterile Solution) have not been conducted.
Teratogenic Effects - Pregnancy Category C.
Morphine sulfate is not teratogenic in rats at 35 mg/kg/day (thirty-five times the usual human dose) but does result in increased pup mortality and growth retardation at doses that narcotize the animal (>10 mg/kg/day, ten times the usual human dose). INFUMORPH (Preservative-free Morphine Sulfate Sterile Solution) should only be given to pregnant women when no other method of controlling pain is available and means are at hand to manage the delivery and perinatal care of the opiate‑dependent infant.
INFUMORPH 200 and 500 (10 and 25 mg/mL, respectively) are too highly concentrated for routine use in obstetric neuraxial analgesia.
Morphine is excreted in maternal milk. Effects on the nursing infant are not known.
Adequate studies, to establish the safety and effectiveness of spinal morphine in pediatric patients, have not been performed, and usage in this population is not recommended.
The pharmacodynamic effects of neuraxial morphine in the elderly are more variable than in the younger population. Patients will vary widely in the effective initial dose, rate of development of tolerance and the frequency and magnitude of associated adverse effects as the dose is increased. Initial doses should be based on careful clinical observation following “test doses”, after making due allowances for the effects of the patient’s age and infirmity on their ability to clear the drug, particularly in patients receiving epidural morphine.
Elderly patients may be more susceptible to respiratory depression and/or respiratory arrest following administration of morphine.
|
IMPROPER OR ERRONEOUS SUBSTITUTION OF INFUMORPH 200 or 500 (10 or 25 mg/mL, respectively) FOR REGULAR DURAMORPH (0.5 or 1 mg/mL) IS LIKELY TO RESULT IN SERIOUS OVERDOSAGE, LEADING TO SEIZURES, RESPIRATORY DEPRESSION AND, POSSIBLY, FATAL OUTCOME. |
The most serious adverse experiences encountered during continuous intrathecal or epidural infusion of INFUMORPH are respiratory depression, myoclonus, and formation of inflammatory masses.
- Single-dose neuraxial administration may result in acute or delayed respiratory depression for periods at least as long as 24 hours. Severe respiratory depression, potentially life-threatening, can result from technical errors during refill, e.g., injection of INFUMORPH outside the filling port, unintentional injection into the direct bypass-dosing port featured on some devices or local infiltration.
- Tolerance and myoclonus: See WARNINGS for discussion of these and related hazards.
- Inflammatory masses: See WARNINGS .
While low doses of intravenously administered morphine have little effect on cardiovascular stability, high doses are excitatory, resulting from sympathetic hyperactivity and increase in circulating catecholamines. Excitation of the central nervous system, resulting in convulsions, may accompany high doses of morphine given intravenously. Dysphoric reactions may occur after any size dose and toxic psychoses have been reported.
Single-dose epidural or intrathecal administration is accompanied by a high incidence of pruritus that is dose-related but not confined to the site of administration. Pruritus, following continuous infusion of epidural or intrathecal morphine, is occasionally reported in the literature; these reactions are poorly understood as to their cause.
Urinary retention, which may persist 10 to 20 hours following single epidural or intrathecal administration, is a frequent side effect and must be anticipated primarily in male patients, with a somewhat lower incidence in females. Also frequently reported in the literature is the occurrence of urinary retention during the first several days of hospitalization for the initiation of continuous intrathecal or epidural morphine therapy. Patients who develop urinary retention have responded to cholinomimetic treatment and/or judicious use of catheters (see PRECAUTIONS ).
Constipation is frequently encountered during continuous infusion of morphine; this can usually be managed by conventional therapy.
Lumbar puncture-type headache is encountered in a significant minority of cases for several days following intrathecal catheter implantation; this, generally, responds to bed rest and/or other conventional therapy.
There are several reports of peripheral edema, including unexplained genital swelling in male patients, following infusion-device implant surgery.
Other adverse experiences reported following morphine therapy include — Dizziness, euphoria, anxiety, depression of cough reflex, interference with thermal regulation and oliguria. Evidence of histamine release such as urticaria, wheals and/or local tissue irritation may occur.
Pruritus, nausea/vomiting and urinary retention, if associated with continuous infusion therapy, may respond to intravenous administration of a low dose of naloxone (0.2 mg). The risks of using narcotic antagonists in patients chronically receiving narcotic therapy should be considered.
|
NALOXONE INJECTION AND RESUSCITATIVE EQUIPMENT SHOULD BE IMMEDIATELY AVAILABLE FOR USE IN CASE OF LIFE-THREATENING OR INTOLERABLE SIDE EFFECTS AND WHENEVER INFUMORPH THERAPY IS BEING INITIATED, THE RESERVOIR IS BEING REFILLED OR ANY MANIPULATION OF THE RESERVOIR SYSTEM IS TAKING PLACE. |
Morphine sulfate is a Schedule II narcotic under the United States Controlled Substance Act (21 U.S.C. 801-886).
Morphine is the most commonly cited prototype for narcotic substances that possess an addiction-forming or addiction-sustaining liability. A patient may be at risk for developing a dependence to morphine if used improperly or for overly long periods of time. As with all potent opioids which are µ-agonists, tolerance as well as psychological and physical dependence to morphine may develop irrespective of the route of administration (intravenous, intramuscular, intrathecal, epidural or oral). Individuals with a prior history of opioid or other substance abuse or dependence, being more apt to respond to the euphorigenic and reinforcing properties of morphine, would be considered to be at greater risk.
Care must be taken to avert withdrawal in patients who have been maintained on parenteral/oral narcotics when epidural or intrathecal administration is considered. Withdrawal symptoms may occur when morphine is discontinued abruptly or upon administration of a narcotic antagonist.
PARENTERAL ADMINISTRATION OF NARCOTICS IN PATIENTS RECEIVING EPIDURAL OR INTRATHECAL MORPHINE MAY RESULT IN OVERDOSAGE.
Overdosage of morphine is characterized by respiratory depression, with or without concomitant CNS depression. In severe overdosage, apnea, circulatory collapse, cardiac arrest and death may occur. Since respiratory arrest may result either through direct depression of the respiratory center, or as the result of hypoxia, primary attention should be given to the establishment of adequate respiratory exchange through provision of a patent airway and institution of assisted, or controlled, ventilation. The narcotic antagonist, naloxone, is a specific antidote. An initial dose of 0.4 to 2 mg of naloxone should be administered intravenously, simultaneously with respiratory resuscitation. If the desired degree of counteraction and improvement in respiratory function is not obtained, naloxone may be repeated at 2- to 3-minute intervals. If no response is observed after 10 mg of naloxone has been administered, the diagnosis of narcotic-induced, or partial narcotic-induced, toxicity should be questioned. Intramuscular or subcutaneous administration may be used if the intravenous route is not available.
As the duration of effect of naloxone is considerably shorter than that of epidural or intrathecal morphine, repeated administration may be necessary. Patients should be closely observed for evidence of renarcotization.
INFUMORPH 200 AND 500 (10 AND 25 MG/ML, RESPECTIVELY) SHOULD NOT BE USED FOR SINGLE-DOSE NEURAXIAL INJECTION BECAUSE LOWER DOSES CAN BE MORE RELIABLY ADMINISTERED WITH THE STANDARD PREPARATION OF DURAMORPH (0.5 AND 1 MG/ML).
CANDIDATES FOR NEURAXIAL ADMINISTRATION OF INFUMORPH IN A CONTINUOUS MICROINFUSION DEVICE SHOULD BE HOSPITALIZED TO PROVIDE FOR ADEQUATE PATIENT MONITORING DURING ASSESSMENT OF RESPONSE TO SINGLE DOSES OF INTRATHECAL OR EPIDURAL MORPHINE. HOSPITALIZATION SHOULD BE MAINTAINED FOR SEVERAL DAYS AFTER SURGERY INVOLVING THE INFUSION DEVICE FOR ADDITIONAL MONITORING AND ADJUSTMENT OF DAILY DOSAGE. THE FACILITY MUST BE EQUIPPED WITH RESUSCITATIVE EQUIPMENT, OXYGEN, NALOXONE INJECTION AND OTHER RESUSCITATIVE DRUGS. BECAUSE OF THE RISK OF DELAYED RESPIRATORY DEPRESSION, PATIENTS SHOULD BE OBSERVED IN A FULLY EQUIPPED AND STAFFED ENVIRONMENT FOR AT LEAST 24 HOURS AFTER EACH TEST DOSE AND, AS INDICATED, FOR THE FIRST SEVERAL DAYS AFTER SURGERY.
Familiarization with the continuous microinfusion device is essential. The desired amount of morphine should be withdrawn from the ampul through a microfilter. To minimize risk from glass or other particles, the product must be filtered through a 5 µ (or smaller) microfilter before injecting into the microinfusion device. If dilution is required, 0.9% Sodium Chloride Injection is recommended.
The starting dose must be individualized, based upon in‑hospital evaluation of the response to serial single-dose intrathecal bolus injections of regular DURAMORPH (Morphine Sulfate Injection, USP) 0.5 mg/mL or 1 mg/mL, with close observation of the analgesic efficacy and adverse effects prior to surgery involving the continuous microinfusion device.
The recommended initial lumbar intrathecal dose range in patients with no tolerance to opioids is 0.2 to 1 mg/day. The published range of doses for individuals who have some degree of opioid tolerance varies from 1 to 10 mg/day. The upper daily dosage limit for each patient must be individualized.
Limited experience with continuous intrathecal infusion of morphine has shown that the daily doses have to be increased over time. Although the rate of increase, over time, in the dose required to sustain analgesia is highly variable, an estimate of the expected rate of increase is shown in the following Figure.

Doses above 20 mg/day should be employed with caution since they may be associated with a higher likelihood of serious side effects (see WARNINGS concerning potential neurological hazards and ADVERSE REACTIONS ).
GERIATRIC:
Administer with extreme caution. (See PRECAUTIONS .)
The starting dose must be individualized, based upon in-hospital evaluation of the response to serial single-dose epidural bolus injections of regular DURAMORPH (Morphine Sulfate Injection, USP) 0.5 mg/mL or 1 mg/mL, with dose observation for analgesic efficacy and adverse effects prior to surgery involving the continuous microinfusion device.
The recommended initial epidural dose in patients who are not tolerant to opioids ranges from 3.5 to 7.5 mg/day. The usual starting dose for continuous epidural infusion, based upon limited data in patients who have some degree of opioid tolerance, is 4.5 to 10 mg/day. The dose requirements may increase significantly during treatment, frequently to 20-30 mg/day. The upper daily limit for each patient must be individualized.
GERIATRIC USE:
Administer with extreme caution. (See PRECAUTIONS .)
|
INFUMORPH is supplied in sealed ampuls. Accidental dermal exposure should be treated by the removal of any contaminated clothing and rinsing the affected area with water. Each ampul of INFUMORPH contains a large amount of a potent narcotic which has been associated with abuse and dependence among health care providers. Due to the limited indications for this product, the risk of overdosage and the risk of its diversion and abuse, it is recommended that special measures be taken to control this product within the hospital or clinic. INFUMORPH should be subject to rigid accounting, rigorous control of wastage and restricted access. This parenteral drug product must be inspected for particulate matter before opening the amber ampul and again for color after removing contents from the ampul. Do not use if the solution in the unopened ampul contains a precipitate which does not disappear upon shaking. After removal, do not use unless the solution is colorless or pale yellow. |
Amber ampuls for epidural or intrathecal administration via a continuous microinfusion device.
INFUMORPH 200 (Preservative-free Morphine Sulfate Sterile Solution)
200 mg/20 mL (10 mg/mL) packaged individually (NDC 0641-6039-01)
INFUMORPH 500 (Preservative-free Morphine Sulfate Sterile Solution)
500 mg/20 mL (25 mg/mL) packaged individually (NDC 0641-6040-01)
Also available from West-Ward: DURAMORPH (Morphine Sulfate Injection, USP)
5 mg/10 mL (0.5 mg/mL) and 10 mg/10 mL (1 mg/mL).
Protect from light. Store at 20° - 25°C (68° - 77°F), excursions permitted to 15°‑30°C (59°‑86°F) [See USP Controlled Room Temperature] until ready to use. DO NOT FREEZE. INFUMORPH contains no preservative or antioxidant. DISCARD ANY UNUSED PORTION. DO NOT HEAT-STERILIZE.
To report SUSPECTED ADVERSE REACTIONS, contact West-Ward Pharmaceutical Corp. at 1-877-845-0689, or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
For Product Inquiry call 1-877-845-0689.
Manufactured by:

WEST-WARD
PHARMACEUTICALS
Eatontown, NJ 07724 USA
Revised September 2011
462-217-01
PRINCIPAL DISPLAY PANEL
INFUMORPH 200 (Preservative-free Morphine Sulfate Sterile Solution)
CII
200 mg/20 mL (10 mg/mL)
20 mL Ampul
NDC 0641-6039-01

INFUMORPH 500 (Preservative-free Morphine Sulfate Solution)
CII
500 mg/20 mL (25 mg/mL)
20 mL Ampul
NDC 0641-6040-01
INFUMORPH 200morphine sulfate INJECTION, SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
INFUMORPH 500morphine sulfate INJECTION, SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||