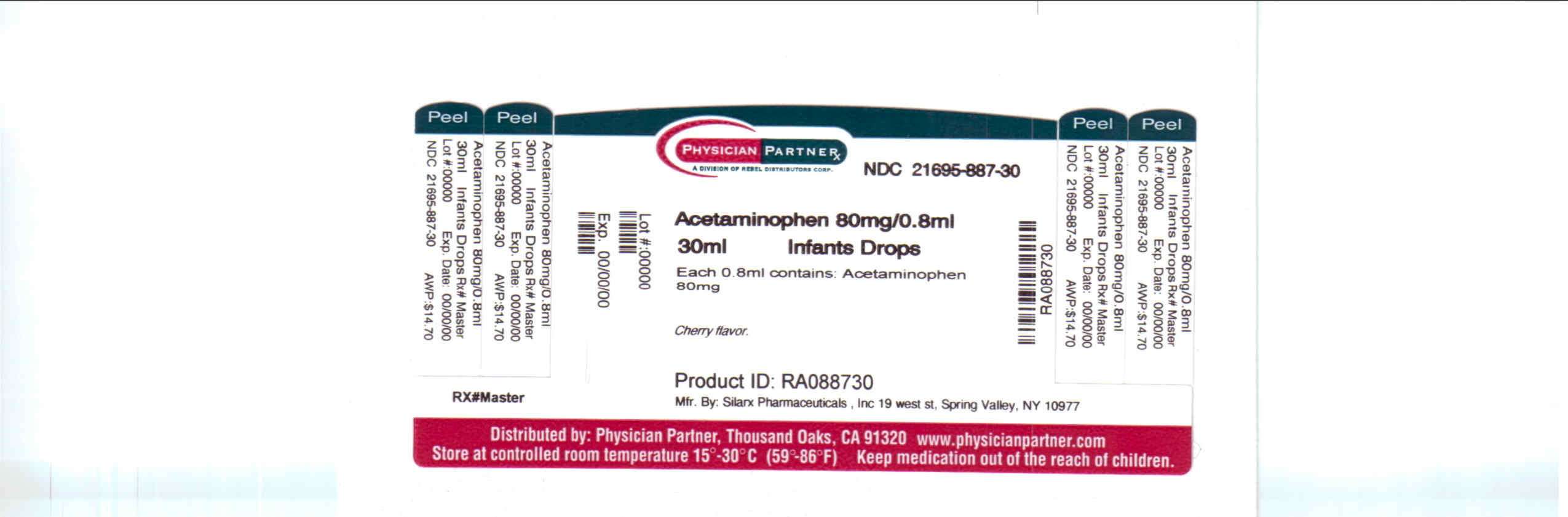
Infants Silapap
Infant's Silapap Drops
FULL PRESCRIBING INFORMATION: CONTENTS*
- Infants Silapap Uses
- Warnings
- Do not use
- When using this product
- Stop use and ask a doctor if
- Directions
- Inactive ingredients
- Comments
FULL PRESCRIBING INFORMATION
Active ingredient
Active Ingredient: Acetaminophen 80 mg (in each 0.8 mL)
Purpose
Purpose: Fever reducer/pain reliever
Infants Silapap Uses
temporarily:
■ reduces fever
■ relieves minor aches and pains due to:
■the common cold ■flu ■toothaches ■sore throat ■headaches
Warnings
Liver warning:
This product contains acetaminophen. Severe liver damage may occur if your child takes:
- more than 5 doses in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
Sore throat warning: if sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
- with any other product containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if your child is allergic to acetaminophen or any of the inactive ingredients in this product
Ask a doctor before use if your child has
liver disease.
Ask a doctor or pharmacist before use if your child is
taking the blood thinning drug warfarin.
When using this product
- do not exceed recommended dosage. (see overdose warning)
Stop use and ask a doctor if
■ new symptoms occur
■ redness or swelling is present
■ pain gets worse or lasts for more than 5 days
■ fever gets worse or lasts for more than 3 days.
These could be signs of serious condition.
Keep out of reach of children.
Overdose warning: Taking more than the recommended dose (overdose) may cause liver damage. In case of overdose, get medical help or contact a Poison Control Center right away, (1-800-222-1222). Quick medical attention is critical even if you do not notice any signs or symptoms.
Directions
■ this product does not contain directions or complete warnings for adult use
■ do not give more than directed (see overdose warning)
■ find right dose on chart, if possible use weight to dose; otherwise, use age
■ use only enclosed dropper designed for use with this product, do not use any other dosing device
■ fill to dose level
■ dispense liquid slowly into child's mouth, toward inner cheek
■ may be given alone or mixed with formula, milk, juice etc.
■ If needed, repeat dose every 4 hours
■ do not give more than 5 times in 24 hours
■ Replace dropper tightly to maintain child resistance
| Weight (lb) |
Age (yr) |
Dose (mL) |
| under 24 |
under 2 years |
ask a doctor |
| 24-35 |
2-3 years |
1.6 mL (0.8 + 0.8 mL) |
For accurate dosing follow dosing instructions using the enclosed dropper. Fill dropper to 0.8 mL or prescribed level, and dispense with a single firm squeeze of the dropper bulb.
Other information
Store between 20° - 25°C (68° - 77°F)
Inactive ingredients
citric acid, FD&C yellow no. 6, cherry flavor, methylparaben, saccharin sodium, sodium benzoate, sodium citrate, propylene glycol and purified water.
Comments
1-888-974-5279
Manufactured by:
Silarx Pharmaceuticals, Inc.
19 West St.
Spring Valley , NY 10977
Repackaged by:
Rebel Distributors Corp
Thousand Oaks, CA 91320
Infants SilapapAcetaminophen SOLUTION/ DROPS
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||