Indomethacin
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- INDOMETHACIN DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- INDOMETHACIN CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- DRUG & OR LABORATORY TEST INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- LABOR & DELIVERY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- INDOMETHACIN ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- SPL MEDGUIDE
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
BOXED WARNING
Cardiovascular RiskNSAIDs may cause an increased risk of serious cardiovascular thrombotic events, myocardial infarction, and stroke, which can be fatal. This risk may increase with duration of use. Patients with cardiovascular disease or risk factors for cardiovascular disease may be at a greater risk. (See WARNINGS.)
Indomethacin is contraindicated for the treatment of peri-operative pain in the setting of coronary artery bypass graft (CABG) surgery (see WARNINGS).
Gastrointestinal Risk
NSAIDs cause an increased risk of serious gastrointestinal adverse events including bleeding, ulceration, and perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Elderly patients are at greater risk for serious gastrointestinal events. (See WARNINGS.)
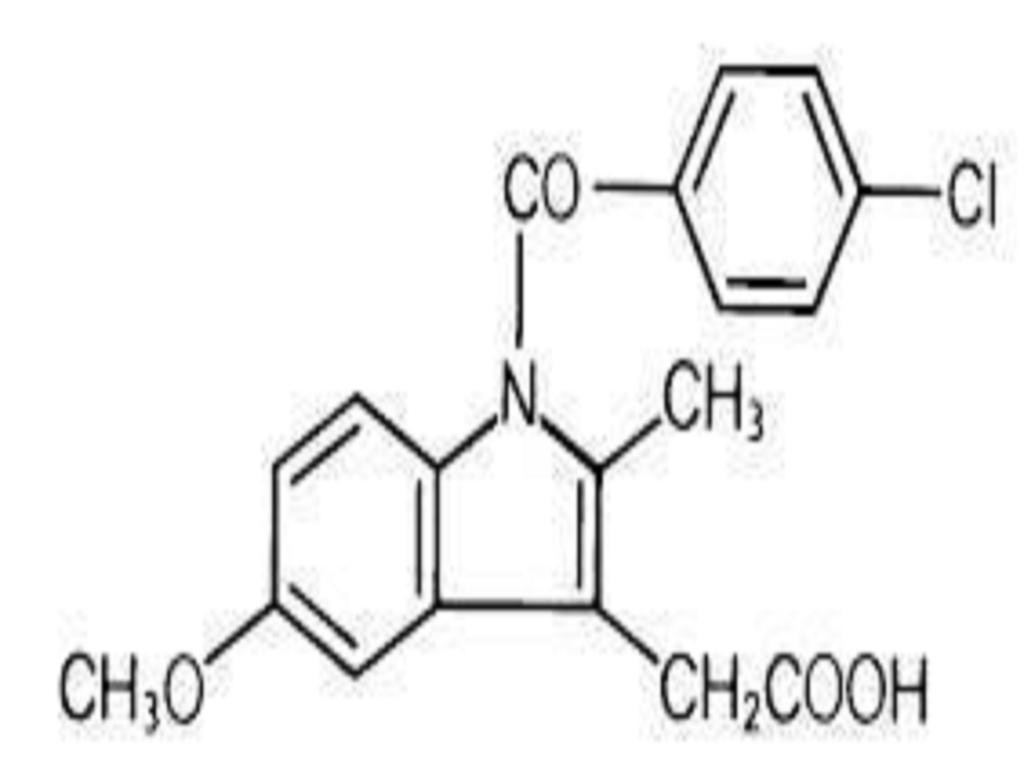
INDOMETHACIN DESCRIPTION
CLINICAL PHARMACOLOGY
INDICATIONS & USAGE
INDOMETHACIN CONTRAINDICATIONS
WARNINGS
PRECAUTIONS
General
INFORMATION FOR PATIENTS
LABORATORY TESTS
DRUG INTERACTIONS
DRUG & OR LABORATORY TEST INTERACTIONS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Teratogenic Effects
Nonteratogenic Effects
LABOR & DELIVERY
NURSING MOTHERS
PEDIATRIC USE
GERIATRIC USE
INDOMETHACIN ADVERSE REACTIONS
OVERDOSAGE
DOSAGE & ADMINISTRATION
HOW SUPPLIED
STORAGE AND HANDLING
SPL MEDGUIDE
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION


IndomethacinIndomethacin CAPSULE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!