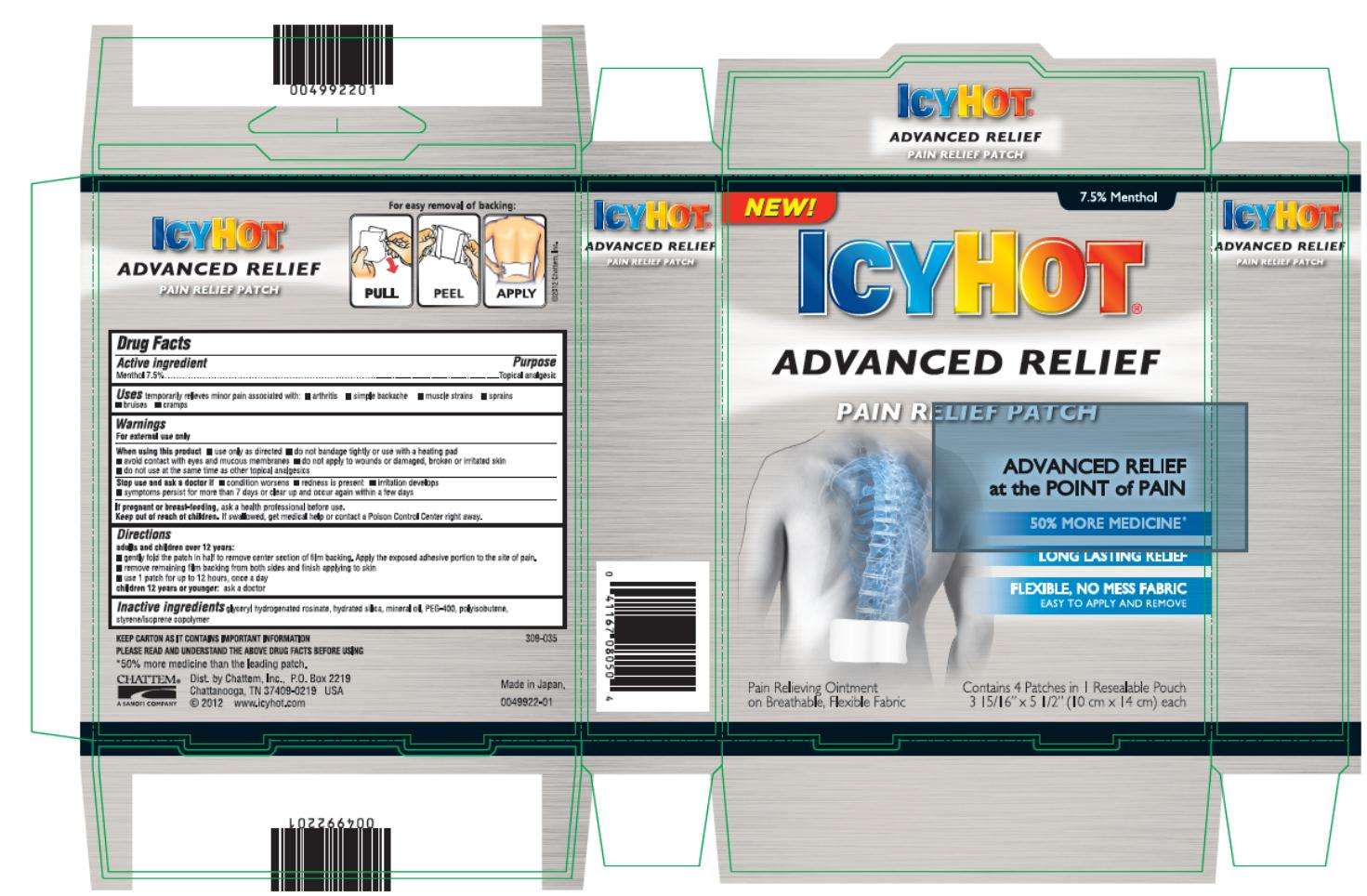
Icy Hot Advanced Relief
Icy Hot Advanced Relief
FULL PRESCRIBING INFORMATION
Menthol 7.5%
Topical analgesic
Temporarily relieves minor pain associated with:
- arthritis
- simple backache
- muscle strains
- sprains
- bruises
- cramps
For external use only
- use only as directed
- do not bandage tightly or use with a heating pad
- avoid contact with eyes and mucous membranes
- do not apply to wounds or damaged, broken or irritated skin
- do not use at the same time as other topical analgesics
- condition worsens
- redness is present
- irritation develops
- symptoms persist for more than 7 days or clear up and occur again within a few days
ask a health professional before use.
If swallowed, get medical help or contact a Poison Control Center right away.
a dults and children over 12 years:
- gently fold the path in half to remove center section of film backing. Apply the exposed adhesive portion to the site of pain.
- remove remaining film backing from both sides and finish applying to skin
- use 1 patch for up to 12 hours, once a day
Children 12 years and younger: ask a doctor
glyceryl hydrogenated rosinate, hydrated silica, mineral oil, PEG-400, polyisobutene, styrne/isoprene copolymer
ICY HOT®
ADVANCED RELIEF
PAIN RELIEF PATCH
ADVANCED RELIEF at the POINT of PAIN
50% MORE MEDICINE
LONG LASTING RELIEF
FLEXIBLE, NO MESS FABRIC
Easy to Apply and Remove
Contains 4 Patches in 1 Resalable Pouch
3 15/16” x 5 ½” (10 cm x 14 cm) each
Icy Hot Advanced ReliefMenthol Topical Analgesic PATCH
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||