I-MAX EXCELLENCE
MAXLIFE USA, INC.
MAXLIFE USA, INC.
I-MAX EXCELLENCE
FULL PRESCRIBING INFORMATION
Active ingredient
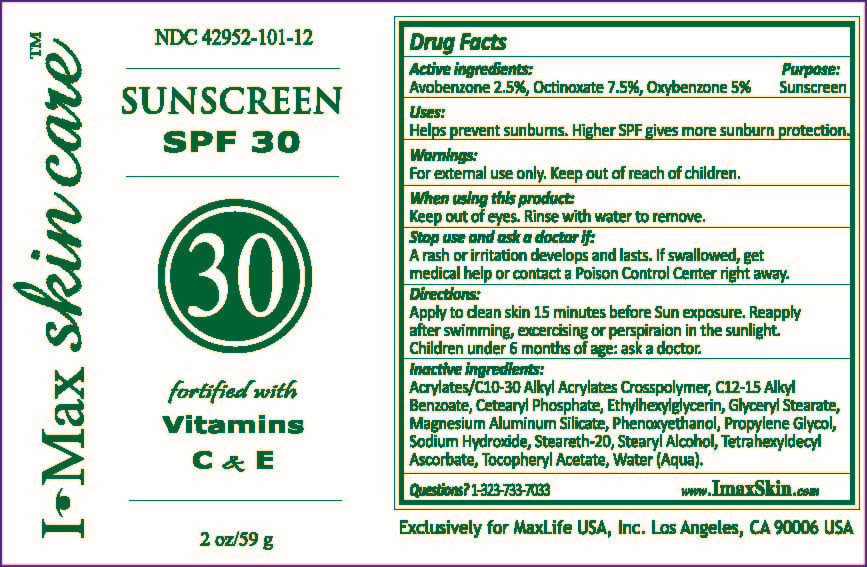
ACTIVE INGREDIENTS:
AVOBENZONE 2.5%
OCTINOXATE 7.5%
OXYBENZONE 5%
Purpose
PURPOSE:
SUNSCREEN
Uses
USES:
HELPS PREVENT SUNBURNS. HIGHER SPF GIVES MORE SUNBURN PROTECTION.
WHEN USING THIS PRODUCT
KEEP OUT OF EYES. RINSE WITH WATER TO REMOVE.
STOP USE AND ASK A DOCTOR IF
A RASH OR IRRITATION DEVELOPS AND LASTS. IF SWALLOWED, GET
MEDICAL HELP OR CONTACT A POISON CONTROL CENTER RIGHT AWAY.
DIRECTIONS
APPLY TO CLEAN SKIN 15 MINUTES BEFORE SUN EXPOSURE. REAPPLY
AFTER SWIMMING, EXCERCISING OR PERSPIRATION IN THE SUNLIGHT.
CHILDREN UNDER 6 MONTHS OF AGE: ASK A DOCTOR.
INACTIVE INGREDIENTS:
ALKYL BENZOATE, ACRYLATES/C10-30 ALKYL ACRYLATES CROSSPOLYMER, C12-15 GLYCERYL STEARATE, CETEARYL PHOSPHATE, ETHYLHEXYLGLYCERIN, MAGNESIUM ALUMINUM SILICATE, PHENOXYETHANOL, PROPYLENE GLYCOL, SODIUM HYDROXIDE, STEARETH-20, STEARYL ALCOHOL, TETRAHEXYLDECYL ASCORBATE, TOCOPHERYL ACETATE, WATER (AQUA).
QUESTIONS? 1-323-733-7033
I-MAX
EXCELLENCE
SPF 30
SUNSCREEN
SPF 30 FOR SKIN PROTECTION
2 FL OZ / 59 ML

I-MAX EXCELLENCEAVOBENZONE OCTINOXATE OXYBENZONE LOTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||