HYPAQUE - CYSTO
HYPAQUE-CYSTO™(Diatrizoate Meglumine Injection, USP)30%
FULL PRESCRIBING INFORMATION: CONTENTS*
- HYPAQUE - CYSTO DESCRIPTION
- CLINICAL PHARMACOLOGY
- HYPAQUE - CYSTO INDICATIONS AND USAGE
- HYPAQUE - CYSTO CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- HYPAQUE - CYSTO ADVERSE REACTIONS
- HYPAQUE - CYSTO DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
FULL PRESCRIBING INFORMATION
Sterile Aqueous Injection
| NOT FOR INTRATHECAL USE |
Not For Intravascular Use
For Retrograde Cystourethrography
Rx ONLY
HYPAQUE - CYSTO DESCRIPTION
HYPAQUE-CYSTO, brand of diatrizoate meglumine, is a water-soluble radiopaque diagnostic medium. It is a triiodinated benzoic acid derivative. It is constituted as a radiopaque iodinated anion (diatrizoate) and a radiolucent cation (meglumine). It is a colorless, microcrystalline solid which is readily soluble in water.
HYPAQUE-CYSTO is a sterile aqueous solution containing 30 g (w/v) of the meglumine salt of diatrizoic acid per 100 mL aqueous solution. The sterile solution is clear and colorless to pale yellow. The pH is adjusted between 6.5 and 7.7 with hydrochloric acid, or diatrizoic acid, or meglumine. It does not contain an antibacterial preservative. It is relatively thermostable and may be autoclaved. Edetate calcium disodium 1:10,000 has been added as a sequestering stabilizing agent. Each 1 mL contains approximately 141 mg of organically bound iodine.
It has an osmolality of 633 mosm/kg (determined by VPO) and is hypertonic to blood.
The viscosity of the solution is 1.94 cp at 25°C and 1.42 cp at 37°C.
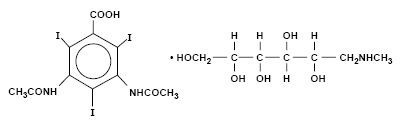
HYPAQUE-CYSTO is a 30 percent solution of 1-Deoxy-l (methylamino)-D-glucitol 3,5-diacetamido-2,4,6-triiodobenzoate (C11H9I3N2O4 • C7H17NO5) with a molecular weight of 809.13, and has the following structural formula:
CLINICAL PHARMACOLOGY
Retrograde introduction of HYPAQUE-CYSTO solution provides radiopacity of the contents of the urinary bladder. When used during micturation as a function test, it also opacifies the bladder neck and lower urinary tract. Continuous fluoroscopic and monitoring of urinary bladder contractions will demonstrate cystoureteric reflux and its extent, if present.
HYPAQUE-CYSTO is not absorbed from the urinary tract to any extent (< 2°), therefore, systemic effects are rare. However, pyelorenal intravasation (especially in patients with ureteric reflux) can occur. Therefore, the potential for adverse effects, such as occur with intravascular use, are possible.
At physiologic pH, the water-soluble contrast media are completely dissociated into a radiopaque anion and a solubilizing cation.
EXCRETION
HYPAQUE-CYSTO which gains inadvertent intravascular entry is not metabolized but excreted unchanged in the urine, each diatrizoate molecule remaining "obligated" to its cation moiety.
Diatrizoate solutions may be excreted either through the kidneys or the liver. These two excretory pathways are not mutually exclusive, but the main route of excretion seems to be governed by the affinity of the contrast medium for serum albumin. From 0% to 10% of diatrizoate meglumine is bound to serum protein.
Diatrizoate salts are excreted unchanged predominantly through the kidneys by glomerular filtration. The amount excreted during any period of time is determined by the filtered load; ie, the product of plasma contrast media concentration and glomerular filtration rate.
The liver and small intestine provide the major alternate route of excretion for diatrizoate. In patients free of severe renal disease, the fecal recovery is less than 2 percent. In patients with severe renal impairment the excretion of these contrast media through the gallbladder and into the small intestine sharply increases; up to 20 percent in the feces in 48 hours.
Saliva is a minor secretory pathway for injectable radiopaque diagnostic agents. In patients with normal renal function, minimal amounts of contrast media are secreted unchanged.
PREGNANCY AND LACTATION
Diatrizoate meglumine crosses the human placental barrier by simple diffusion and appears to enter fetal tissues passively. No apparent harm to the fetus occurs. Procedures including radiation involve a certain risk related to the exposure of the fetus.
Diatrizoate solutions are excreted unchanged in human milk.
HYPAQUE - CYSTO INDICATIONS AND USAGE
HYPAQUE-CYSTO is indicated for retrograde cystourethrography in adult and pediatric patients.
HYPAQUE - CYSTO CONTRAINDICATIONS
HYPAQUE-CYSTO has no absolute contraindication in its recommended use.
WARNINGS
SEVERE ADVERSE EVENTS—INADVERTENT INTRATHECAL USE
Serious adverse reactions have been reported due to the inadvertent intrathecal administration of iodinated contrast media that are not indicated for intrathecal use. These serious adverse reactions include: death, convulsions, cerebral hemorrhage, coma, paralysis, arachnoiditis, acute renal failure, cardiac arrest, seizures, rhabdomyolysis, hyperthermia, and brain edema. Special attention must be given to insure that this drug product is not administered intrathecally.
Ionic iodinated contrast media inhibit blood coagulation, in vitro, more than nonionic contrast media. Nonetheless, it is prudent to avoid prolonged contact of blood with syringes containing ionic contrast media.
Serious, rarely fatal, thromboembolic events causing myocardial infarction and stroke have been reported during angiographic procedures with both ionic and nonionic contrast media. Therefore, meticulous intravascular administration technique is necessary, particularly during angiographic procedures, to minimize thromboembolic events. Numerous factors, including length of procedure, catheter and syringe material, underlying disease state and concomitant medications may contribute to the development of thromboembolic events. For these reasons, meticulous angiographic techniques are recommended including close attention to guidewire and catheter manipulation, use of manifold systems and/or three-way stopcocks, frequent catheter flushing with heparinized saline solutions and minimizing the length of the procedure. The use of plastic syringe in place of glass syringes has been reported to decrease but not eliminate the likelihood of in vitro clotting.
Serious or fatal reactions have been associated with the vascular entry of radiopaque media. It is important that a course of action be carefully planned in advance for the treatment of possible serious reactions.
PRECAUTIONS
General
Diagnostic procedures which involve the use of radiopaque diagnostic agents should be carried out under the direction of personnel with the prerequisite training and with a thorough knowledge of the particular procedure to be performed. Appropriate facilities should be available for the management of any complication of the procedure, as well as for emergency treatment of severe reactions to the contrast agent itself. Competent personnel and emergency facilities should be available for at least 30 to 60 minutes since severe delayed reactions have occurred (See ADVERSE REACTIONS).
The possibility of a reaction, including serious, life-threatening, fatal, anaphylatic or cardiovascular reactions should always be considered (see ADVERSE REACTIONS). It is of utmost importance that a course of action be carefully planned in advance for immediate treatment of serious reactions, and that adequate and appropriate personnel be readily available in case of any reaction.
ALLERGIC HISTORY
Before injecting a contrast medium, the patient should be questioned for a history of allergy. A positive history does not arbitrarily contraindicate the use of a contrast agent where a diagnostic procedure is considered essential, but caution should be exercised (see ADVERSE REACTIONS).
The possibility of an idiosyncratic reaction in susceptible patients should always be considered (see ADVERSE REACTIONS). The susceptible population includes patients with a history of a previous reaction to a contrast media, patients with a known sensitivity to iodine per se, and patients with known clinical hypersensitivity (ie, bronchial asthma, hay fever, and food allergies).
Premedication with antihistamines or corticosteroids to avoid or minimize possible allergic reactions in such patients should be considered. Recent reports indicate that such pretreatment does not prevent serious life-threatening reactions, but may reduce both their incidence and severity.
TEST DOSE
The occurrence of severe idiosyncratic reactions has prompted the use of several pretesting methods. However, pretesting cannot be relied upon to predict severe reactions and may itself be hazardous for the patient. It is suggested that a thorough medical history with emphasis on allergy and hypersensitivity, prior to the injection of any contrast media, may be more accurate than pretesting in predicting adverse reactions.
Information for Patients
Patients receiving injectable radiopaque diagnostic agents should be instructed to:
- Inform the physician if they are pregnant (see CLINICAL PHARMACOLOGY).
- Inform the physician if they are allergic to any drugs, food, or if they have had any reactions to previous injections of dyes used for x-ray procedures (see PRECAUTIONS).
Drug/Laboratory Test Interactions
Under certain circumstances (pH, temperature, concentrations, time), diatrizoate solutions are incompatible with promethazine hydrochloride, diphenhydramine hydrochloride, brompheniramine maleate, or papaverine hydrochloride solutions.
BENADRYL®, brand of diphenhydramine hydrochloride, may cause precipitation when mixed in the same syringe with HYPAQUE-CYSTO.
Although interference with these laboratory tests have not been reported following cystography absorption (from the bladder or by pyelorenal back flow), they have occurred following direct intravenous injection. Therefore, if any of these studies, which might be affected by contrast media are indicated, it is recommended that they be performed prior to administration of the contrast medium or two or more days afterwards.
Diatrizoate salts interfere with several laboratory urine and blood tests.
Blood Tests
Coagulation: Diatrizoate salts significantly inhibit all stages of coagulation. The fibrinogen concentration, Factors V, VII, and VIII are decreased. Prothrombin time and thromboplastin time are increased.
Platelet aggregation: High levels of plasma and diatrizoate meglumine inhibit platelet aggregation.
Serum calcium: Diatrizoate salts may decrease serum calcium levels. However, this depletion of serum calcium may also be the result of the addition of chelating agents (edetate disodium) in the preparation of certain contrast media.
Red cell counts: Transitory decreases in red cell counts. Technetium-99m-RBC labeling interference.
Leukocyte counts: Decrease.
Urea nitrogen (BUN): Transitory increase (see CLINICAL PHARMACOLOGY).
Serum creatinine: Transitory increase.
Urine Tests
Urine osmolarity and specific gravity. Decreased due to induced diuresis.
Urine cultures. Diatrizoate in urine cultures may inhibit bacterial growth.
Thyroid Function Tests
Protein-bound iodine (PBI) and total serum organic iodine: Transient increase of both tests following cystography and retrograde pyelography have been noticed. The results of PBl and radioactive iodine uptake studies which depend on iodine estimations will not accurately reflect thyroid function for up to 16 days following administration of iodinated media. However, thyroid function tests not depending on iodine estimations, eg, T3 resin uptake or free thyroxine assays are not affected.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals have not been performed in order to evaluate carcinogenic potential, mutagenesis, or whether HYPAQUE-CYSTO can affect fertility in males or females.
Pregnancy Category C
Animal reproduction studies have not been conducted with diatrizoate meglumine. It is also not known whether it can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Therefore, diatrizoate meglumine should be given to a pregnant woman only if clearly needed.
Labor and Delivery
It is not known whether use of these contrast agents during labor or delivery has immediate or delayed adverse effects on the fetus, prolongs the duration of labor or increases the likelihood that forceps delivery or other obstetrical intervention or resuscitation of the newborn will be necessary.
Nursing Mothers
Diatrizoate salts are excreted unchanged in human milk. Because of the potential adverse reactions, although it has not been established that serious adverse reactions occur in nursing infants, caution should be exercised when these contrast media are administered to a nursing woman.
HYPAQUE - CYSTO ADVERSE REACTIONS
Not For Intravascular Use
Because inadvertent intravascular entry of HYPAQUE-CYSTO is possible during urethrocystography (bladder absorption or pyelorenal back flow), the occurrence of systemic adverse effects is possible. However, the relative incidence and severity of the following reactions refer only to experience with direct intravascular injection.
Approximately 95 percent of adverse reactions accompanying the intravascular use of diatrizoate salts are of mild to moderate severity. However, life-threatening reactions and fatalities, mostly of cardiovascular origin, have occurred.
Adverse reactions to injectable contrast media fall into two categories: chemotoxic reactions and idiosyncratic reactions.
Chemotoxic reactions result from the physicochemical properties of the contrast media, the dose, and the speed of injection. All hemodynamic disturbances and injuries to organs or vessels perfused by the contrast medium are included in this category.
Idiosyncratic reactions include all other reactions. They occur more frequently in patients 20 to 40 years old. Idiosyncratic reactions may or may not be dependent on the amount of dose injected, the speed of injection, the mode of injection, and the radiographic procedure. Idiosyncratic reactions are subdivided into minor, intermediate, and severe. The minor reactions are self-limited and of short duration; the severe reactions are life-threatening and treatment is urgent and mandatory.
The reported incidence of adverse reactions to contrast media in patients with a history of allergy are twice that of the general population. Patients with a history of previous reactions to a contrast medium are three times more susceptible than other patients. However, sensitivity to contrast media does not appear to increase with repeated examinations.
Most adverse reactions to injectable contrast media appear within one to three minutes after the start of injection, but delayed reactions may occur.
Adverse reactions are grouped by organ system and listed below by decreasing order of occurrence and with an approximate incidence of occurrence. Significantly more severe reactions are listed before the other reactions regardless of frequency.
Greater Than 1 in 100 Patients
Body as a Whole: Reported incidences of death range from 6.6 per 1 million (0.00066 percent) to 1 in 10,000 patients (0.01 percent). Most deaths occur during injection or 5 to 10 minutes later, the main feature being cardiac arrest with cardiovascular disease as the main aggravating factor.
Isolated reports of hypotensive collapse and shock following urography are found in the literature. The incidence of shock is estimated to occur in 1 out of 20,000 (0.005 percent) patients.
Cardiovascular System: The most frequent adverse reaction to diatrizoate salts is vasodilation (feeling of warmth). The estimated incidence is 49 percent.
Digestive System: Nausea 6 percent, vomiting 3 percent.
Nervous System: Paresthesia 6 percent, dizziness 5 percent.
Respiratory System: Rhinitis 1 percent, increased cough 2 percent.
Skin and Appendages: Urticaria 1 percent.
Pain at the injection site is estimated to occur in about 12 percent of the patients undergoing urography. Pain is usually due to extravasation.
Painful hot erythematous swelling above the venipuncture site was estimated to occur in more than 1 percent of the patients undergoing phlebography.
Special Senses: Perversion of taste 11 percent.
Urogenital System: Osmotic nephrosis of the proximal tubular cells is estimated to occur in 23 percent of patients following excretory urography.
Less Than 1 in 100 Patients
Other infrequently reported reactions without accompanying incidence rates are listed below, grouped by organ system.
Body as a Whole: Malaria relapse, uremia, high creatinine and BUN (see PRECAUTIONS-Drug/Laboratory Test Interactions), thrombocytopenia, leukopenia, and anemia.
Cardiovascular System: Cerebral hematomas, hemodynamic disturbances, sinus bradycardia, transient electrocardiographic abnormalities, ventricular fibrillation, petechiae, chest pain, and cardiac arrest.
Digestive System: Severe unilateral or bilateral swelling of the parotid and submaxillary glands.
Nervous System: Convulsions, paralysis, and coma.
Respiratory System: Asthma, dyspnea, laryngeal edema, pulmonary edema, and bronchospasm.
Skin and Appendages: Skin necrosis.
Special Senses: Bilateral ocular irritation, lacrimation, itching, conjunctival chemosis, infection, and conjunctivitis.
Urogenital: Renal failure, pain.
HYPAQUE - CYSTO DOSAGE AND ADMINISTRATION
After the bladder is emptied, HYPAQUE-CYSTO is gently instilled without force, often beyond the first desire to micturate, but not beyond the point of urgency or mild discomfort. The volume required to fill the bladder to slightly less than capacity may vary from patient to patient.
Bladder capacity in normal adults is generally 200 mL to 300 mL, and rarely, up to 600 mL. Capacity at birth is 20 mL to 50 mL, and increases about 400 percent in the first year. In children 3 to 5 years old, bladder capacity is 150 mL to 180 mL. In children older than 8 years, it is in the low adult range.
In disease, bladder capacity in adults may vary from 50 mL in a hypertonic reflex bladder to over 1000 mL in an atonic or sensory paralytic bladder or chronic lower urinary tract obstruction.
Repeat examination may be required to detect reflux, or in function studies.
The concentration varies with technique and equipment used. HYPAQUE-CYSTO may be diluted with sterile water or 5 percent dextrose solution, as indicated in the following table. A 10 percent solution is isotonic.
| TO MAKE | ADD | FINAL SOLUTION CONTAINS | |
|---|---|---|---|
| Final conc. | Final volume | Sterile water or 5% dextrose solution | Iodine |
| 30% | 250 mL | _ | 141 mg/mL |
| 25% | 300 mL | 50 mL | 118 mg/mL |
| 21.4% | 350 mL | 100 mL | 101 mg/mL |
| 20% | 375 mL | 125 mL | 94 mg/mL |
| 18.8% | 400 mL | 150 mL | 88 mg/mL |
| 16.7% | 450 mL | 200 mL | 78 mg/mL |
| 15% | 500 mL | 250 mL | 71 mg/mL |
Note: To achieve the following concentrations some of the contrast agent must be removed prior to dilution.
| TO MAKE | REMOVE | ADD | FINAL SOLUTION CONTAINS | |
|---|---|---|---|---|
| Final conc. | Final volume |  | Sterile water or 5% dextrose solution | Iodine |
| 12% | 500 mL | 50 mL | 300 mL | 56 mg/mL |
| 12% | 375 mL | 100 mL | 225 mL | 56 mg/mL |
| 10% | 450 mL | 100 mL | 300 mL | 47 mg/mL |
Dilution and withdrawal of the contrast agent should be accomplished under aseptic conditions with sterile syringes. The solution should be inspected visually for particulate matter and discoloration prior to administration.
HOW SUPPLIED
STANDARD PACKAGE
Calibrated 500 mL dilution bottles containing 250 mL HYPAQUE-CYSTO; rubber stoppered, with hangers, inner removable seal and screw neck. Box of 10 (NDC 0407-0734-10)
Protect from light. Contains no preservatives, therefore, discard any unused portion remaining in the container.
Store at 15°C to 30°C (59°F to 86°F).
Distributed by Amersham Health Inc.
Princeton, NJ 08540
Printed in USA
Revised February 2003
HNC-3D
HYPAQUE - CYSTODiatrizoate Meglumine INJECTION, SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||