HYDROMORPHONE HYDROCHLORIDE
Hydromorphone Hydrochloride CII
FULL PRESCRIBING INFORMATION: CONTENTS*
- HYDROMORPHONE HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- HYDROMORPHONE HYDROCHLORIDE INDICATIONS AND USAGE
- HYDROMORPHONE HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- HYDROMORPHONE HYDROCHLORIDE ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- HYDROMORPHONE HYDROCHLORIDE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PRINCIPAL DISPLAY PANEL - 3 mg Packet Label
FULL PRESCRIBING INFORMATION
HYDROMORPHONE HYDROCHLORIDE DESCRIPTION
Hydromorphone hydrochloride (WARNING: May be habit forming), a hydrogenated ketone of morphine, is a narcotic analgesic. It is available in:
Suppositories (for rectal administration) containing:
-
The structural formula of hydromorphone hydrochloride is:
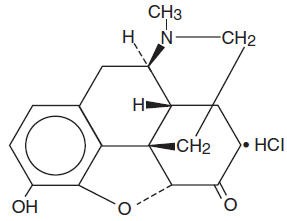
CLINICAL PHARMACOLOGY
Hydromorphone hydrochloride is a narcotic analgesic; its principal therapeutic effect is relief of pain. The precise mechanism of action of hydromorphone hydrochloride and other opiates is not known, although it is believed to relate to the existence of opiate receptors in the central nervous system. There is no intrinsic limit to the analgesic effect of hydromorphone hydrochloride; like morphine, adequate doses will relieve even the most severe pain. Clinically, however, dosage limitations are imposed by the adverse effects, primarily respiratory depression, nausea, and vomiting, which can result from high doses.
Hydromorphone hydrochloride has diverse additional actions. It may produce drowsiness, changes in mood and mental clouding, depress the respiratory center and the cough center, stimulate the vomiting center, produce pinpoint constriction of the pupil, enhance parasympathetic activity, elevate cerebrospinal fluid pressure, increase biliary pressure, produce transient hyperglycemia.
Generally, the analgesic action of parenterally administered hydromorphone hydrochloride is apparent within 15 minutes and usually remains in effect for more than five hours. The onset of action of oral hydromorphone hydrochloride is somewhat slower, with measurable analgesia occurring within 30 minutes.
HYDROMORPHONE HYDROCHLORIDE INDICATIONS AND USAGE
Hydromorphone hydrochloride is indicated for the relief of moderate to severe pain such as that due to:
|
|
HYDROMORPHONE HYDROCHLORIDE CONTRAINDICATIONS
Hydromorphone hydrochloride is contraindicated in patients with a known hypersensitivity to hydromorphone; in the presence of an intracranial lesion associated with increased intracranial pressure; and whenever ventilatory function is depressed (chronic obstructive pulmonary disease, cor pulmonale, emphysema, kyphoscoliosis, status asthmaticus).
WARNINGS
Respiratory Depression:
Hydromorphone hydrochloride produces dose-related respiratory depression by acting directly on brain stem respiratory centers. Hydromorphone hydrochloride also affects centers that control respiratory rhythm, and may produce irregular and periodic breathing.
Head Injury and Increased Intracranial Pressure:
The respiratory depressant effects of narcotics and their capacity to elevate cerebrospinal fluid pressure may be markedly exaggerated in the presence of head injury, other intracranial lesions or a preexisting increase in intracranial pressure. Furthermore, narcotics produce effects which may obscure the clinical course of patients with head injuries.
Acute Abdominal Conditions:
The administration of narcotics may obscure the diagnosis or clinical course of patients with acute abdominal conditions.
PRECAUTIONS
Special Risk Patients:
Hydromorphone hydrochloride should be used with caution in elderly or debilitated patients and those with impaired renal or hepatic function, hypothyroidism, Addison's disease, prostatic hypertrophy or urethral stricture. As with any narcotic analgesic agent, the usual precautions should be observed and the possibility of respiratory depression should be kept in mind.
Cough Reflex:
Hydromorphone hydrochloride suppresses the cough reflex; as with all narcotics, caution should be exercised when hydromorphone hydrochloride is used postoperatively and in patients with pulmonary disease.
Usage in Ambulatory Patients:
Narcotics may impair the mental and/or physical abilities required for the performance of potentially hazardous tasks such as driving a car or operating machinery; patients should be cautioned accordingly.
Drug Interactions:
Patients receiving other narcotic analgesics, general anesthetics, phenothiazines, tranquilizers, sedative-hypnotics, tricyclic antidepressants or other CNS depressants (including alcohol) concomitantly with hydromorphone hydrochloride may exhibit an additive CNS depression. When such combined therapy is contemplated, the dose of one or both agents should be reduced.
Pregnancy:
Pregnancy Category C
Hydromorphone hydrochloride has been shown to be teratogenic in hamsters when given in doses 600 times the human dose. There are no adequate and well-controlled studies in pregnant women. Hydromorphone hydrochloride should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nonteratogenic Effects:
Babies born to mothers who have been taking opioids regularly prior to delivery will be physically dependent. The withdrawal signs include irritablity and excessive crying, tremors, hyperactive reflexes, increased respiratory rate, increased stools, sneezing, yawning, vomiting, and fever. The intensity of the syndrome does not always correlate with the duration of maternal opioid use or dose. There is no consensus on the best method of managing withdrawal. Chlorpromazine 0.7 to 1.0 mg/kg q6h, phenobarbital 2 mg/kg q6h, and paregoric 2 to 4 drops/kg q4h, have been used to treat withdrawal symptoms in infants. The duration of therapy is 4 to 28 days, with the dosages decreased as tolerated.
Labor and Delivery:
As with all narcotics, administration of hydromorphone hydrochloride to the mother shortly before delivery may result in some degree of respiratory depression in the newborn, especially if higher doses are used.
Nursing Mothers:
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from hydromorphone hydrochloride, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use:
Safety and effectiveness in children have not been established.
HYDROMORPHONE HYDROCHLORIDE ADVERSE REACTIONS
Central Nervous System:
Sedation, drowsiness, mental clouding, lethargy, impairment of mental and physical performance, anxiety, fear, dysphoria, dizziness, psychic dependence, mood changes.
Gastrointestinal System:
Nausea, and vomiting occur infrequently; they are more frequent in ambulatory than in recumbent patients. The antiemetic phenothiazines are useful in suppressing these effects; however, some phenothiazine derivatives seem to be antianalgesic and to increase the amount of narcotic required to produce pain relief, while other phenothiazines reduce the amount of narcotic required to produce a given level of analgesia. Prolonged administration of hydromorphone hydrochloride may produce constipation. Opiate agonist-induced increase in intraluminal pressure may endanger surgical anastomosis.
Cardiovascular System:
Circulatory depression, peripheral circulatory collapse and cardiac arrest have occurred after rapid intravenous injection. Orthostatic hypotension and fainting may occur if a patient stands up suddenly after receiving an injection of hydromorphone hydrochloride.
Genitourinary System:
Ureteral spasm, spasm of vesical sphincters and urinary retention have been reported.
Respiratory Depression:
Hydromorphone hydrochloride produces dose-related respiratory depression by acting directly on brain stem respiratory centers. Hydromorphone hydrochloride also affects centers that control respiratory rhythm, and may produce irregular and periodic breathing. If significant respiratory depression occurs, it may be antagonized by the use of naloxone hydrochloride. The usual adult dose of 0.4 to 0.8 mg given intramuscularly or intravenously, promptly reverses the effects of morphine-like opioid agonists such as hydromorphone hydrochloride. In patients who are physically dependent, small doses of naloxone may be sufficient not only to antagonize respiratory depression, but also to precipitate withdrawal phenomena. The dose of naloxone should therefore be adjusted accordingly in such patients. SInce the duration of action of hydromorphone hydrochloride may exceed that of the antagonist, the patient should be kept under continued surveillance; repeated doses of the antagonist may be required to maintain adequate respiration. Apply other supportive measures when indicated.
DRUG ABUSE AND DEPENDENCE
Hydromorphone hydrochloride is a Schedule II narcotic. Psychic dependence, physical dependence, and tolerance may develop upon repeated administration of narcotics; therefore, hydromorphone hydrochloride should be prescribed and administered with caution. However, psychic dependence is unlikely to develop when hydromorphone hydrochloride is used for a short time for the treatment of pain. Physical dependence, the condition in which continued administration of the drug is required to prevent the appearance of a withdrawal syndrome, usually assumes clinically significant proportions only after several weeks of continued narcotic use, although some mild degree of physical dependence may develop after a few days of narcotic therapy. Tolerance, in which increasingly large doses are required in order to produce the same degree of analgesia, is manifested initially by a shortened duration of analgesic effect, and subsequently by decreases in the intensity of analgesia. The rate of development of tolerance varies among patients.
OVERDOSAGE
Signs and Symptoms:
Serious overdosage with hydromorphone hydrochloride is characterized by respiratory depression (a decrease in respiratory rate and/or tidal volume, Cheyne-Stokes respiration, cyanosis), extreme somnolence progressing to stupor or coma, skeletal muscle flaccidity, cold and clammy skin, and sometimes bradycardia and hypotension. In severe overdosage, particularly the intravenous route, apnea, circulatory collapse, cardiac arrest, and death may occur.
Treatment:
Primary attention should be given to the reestablishment of adequate respiratory exchange through provision of a patent airway and institution of assisted or controlled ventilation. The narcotic antagonist naloxone hydrochloride is a specific antidote against respiratory depression which may result from overdosage or unusual sensitivity to narcotics, including hydromorphone hydrochloride. Therefore, naloxone hydrochloride should be administered as described under Adverse Reactions (see Respiratory Depression ) in conjunction with ventilatory assistance.
Since the duration of action of hydromorphone hydrochloride may exceed that of the antagonist, the patient should be kept under continued surveillance; repeated doses of the antagonist may be required to maintain adequate respiration. An antagonist should not be administered in the absence of clinically significant respiratory or cardiovascular depression. Oxygen, intravenous fluids, vasopressors, and other supportive measures should be employed as indicated.
HYDROMORPHONE HYDROCHLORIDE DOSAGE AND ADMINISTRATION
Hydromorphone Hydrochloride Suppositories (3 mg) may provide longer duration of relief which could obviate additional medication during the sleeping hours. The usual adult dose is one (1) suppository inserted rectally every 6 to 8 hours or as directed by physician.
HOW SUPPLIED
Rectal Suppositories: 3 mg Suppositories
-
Hydromorphone Hydrochloride Suppositories should be stored in a refrigerator. Protect from light.
A Schedule CII Narcotic
DEA Order Form Required
Manufactured By
Perrigo
®
Minneapolis, MN 55427
(01-12)
PRINCIPAL DISPLAY PANEL - 3 mg Packet Label
Hydromorphone HCl 3 mg
Suppository
(01)10305747224069
Manufactured By Perrigo®
Minneapolis, MN 55427
CII

HYDROMORPHONE HYDROCHLORIDEHYDROMORPHONE HYDROCHLORIDE SUPPOSITORY
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||