Hydrochlorothiazide
FULL PRESCRIBING INFORMATION: CONTENTS*
- HYDROCHLOROTHIAZIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- HYDROCHLOROTHIAZIDE INDICATIONS AND USAGE
- HYDROCHLOROTHIAZIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- HYDROCHLOROTHIAZIDE ADVERSE REACTIONS
- OVERDOSAGE
- HYDROCHLOROTHIAZIDE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 25 mg (100 Tablet Bottle)
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 50 mg (100 Tablet Bottle)
FULL PRESCRIBING INFORMATION
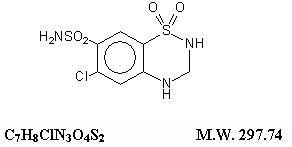
HYDROCHLOROTHIAZIDE DESCRIPTION
H
CLINICAL PHARMACOLOGY
Pharmacokinetics and Metabolism
HYDROCHLOROTHIAZIDE INDICATIONS AND USAGE
Use in Pregnancy
PRECAUTIONS, Pregnancy
HYDROCHLOROTHIAZIDE CONTRAINDICATIONS
WARNINGS
PRECAUTIONS, Drug Interactions
Acute Myopia and Secondary Angle-Closure Glaucoma
PRECAUTIONS
General
Laboratory Tests
Drug Interactions
Drug/Laboratory Test Interactions
PRECAUTIONS, General
Carcinogenesis, Mutagenesis, Impairment of Fertility
in vitroSalmonella typhimuriumin vivoDrosophilain vitroAspergillus nidulans
Pregnancy
Teratogenic Effects
Nonteratogenic Effects
Nursing Mothers
Pediatric Use
DOSAGE AND ADMINISTRATION, Infants and Children
HYDROCHLOROTHIAZIDE ADVERSE REACTIONS
Body as a Whole
Cardiovascular
Digestive
Hematologic
Hypersensitivity
Metabolic
PRECAUTIONS
Musculoskeletal
Nervous System/Psychiatric
Renal
WARNINGS
Skin
Special Senses
Urogenital
OVERDOSAGE
50
HYDROCHLOROTHIAZIDE DOSAGE AND ADMINISTRATION
Adults
For Edema
For Control of Hypertension
PRECAUTIONS
Infants and Children
For Diuresis and for Control of Hypertension
PRECAUTIONS, Pediatric Use
HOW SUPPLIED
Hydrochlorothiazide Tablets USP, 25 mg
Hydrochlorothiazide Tablets USP, 50 mg
PHARMACIST:
Store at
Aurobindo Pharma USA, Inc.
Aurobindo Pharma Limited
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 25 mg (100 Tablet Bottle)
NDC 65862-133-01
Hydrochlorothiazide Tablets, USP
25 mg
Rx only 100 Tablets
AUROBINDO

PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 50 mg (100 Tablet Bottle)
NDC 65862-134-01
Hydrochlorothiazide Tablets, USP
50 mg
Rx only 100 Tablets
AUROBINDO

HydrochlorothiazideHydrochlorothiazide TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
HydrochlorothiazideHydrochlorothiazide TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!