Hydrochlorothiazide
FULL PRESCRIBING INFORMATION: CONTENTS*
- HYDROCHLOROTHIAZIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACOKINETICS
- PHARMACODYNAMICS
- CLINICAL STUDIES
- INDICATIONS & USAGE
- HYDROCHLOROTHIAZIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- DRUG INTERACTIONS
- DRUG & OR LABORATORY TEST INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- HYDROCHLOROTHIAZIDE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- INACTIVE INGREDIENT
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
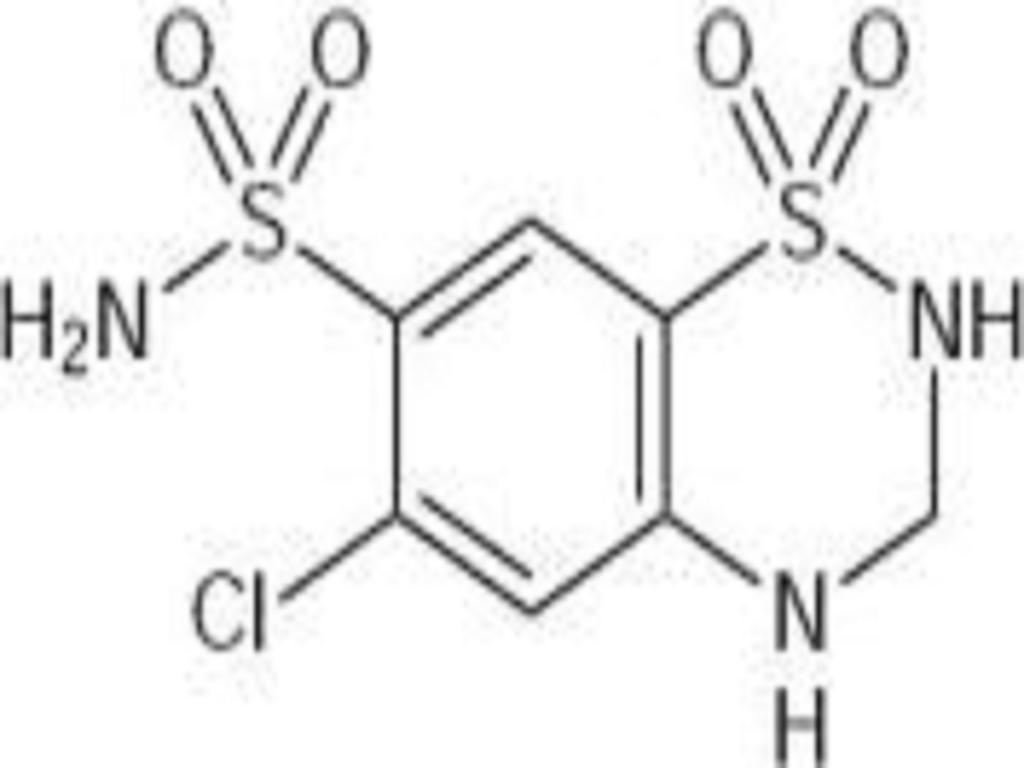
HYDROCHLOROTHIAZIDE DESCRIPTION
CLINICAL PHARMACOLOGY
PHARMACOKINETICS
PHARMACODYNAMICS
CLINICAL STUDIES
INDICATIONS & USAGE
HYDROCHLOROTHIAZIDE CONTRAINDICATIONS
WARNINGS
PRECAUTIONS
DRUG INTERACTIONS
DRUG & OR LABORATORY TEST INTERACTIONS
PRECAUTIONS, GeneralCARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Teratogenic Effects:Nonteratogenic Effects:
NURSING MOTHERS
PEDIATRIC USE
GERIATRIC USE
HYDROCHLOROTHIAZIDE ADVERSE REACTIONS
PRECAUTIONS
WARNINGS
OVERDOSAGE
DOSAGE & ADMINISTRATION
HOW SUPPLIED
INACTIVE INGREDIENT
D&C RED NO. 28D&C YELLOW NO. 10
FD&C BLUE NO. 1
FERRIC OXIDE BLACK
GELATIN
LACTOSE MONOHYDRATE
MAGNESIUM STEARATE
COLLOIDAL SILICON DIOXIDE
SODIUM LAURYL SULFATE
SODIUM STARCH GLYCOLATE TYPE A POTATO
STARCH, CORN
TITANIUM DIOXIDE
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

HydrochlorothiazideHydrochlorothiazide CAPSULE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!