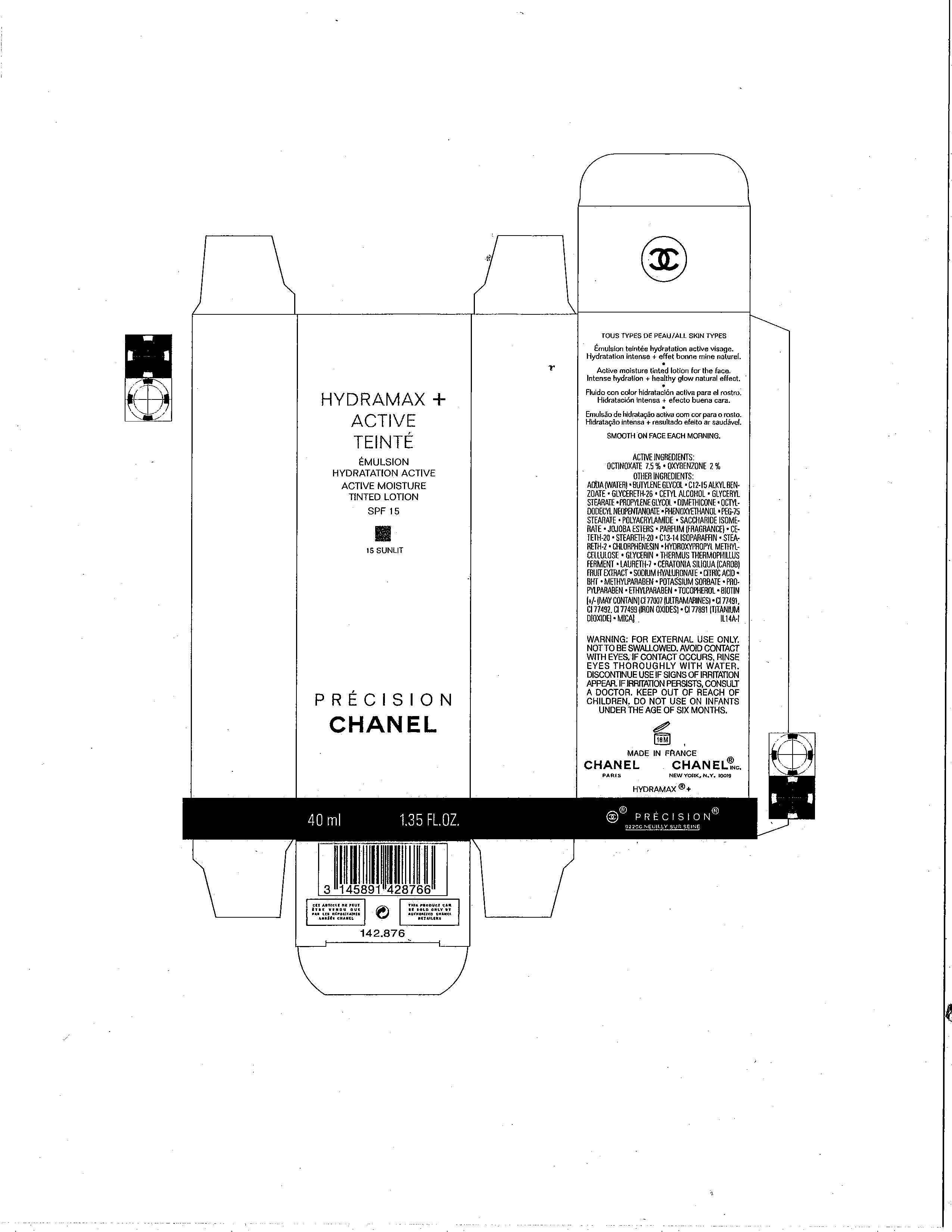
HYDRAMAX ACTIVE TEINTE
DRUG FACTS
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
ACTIVE INGREDIENT(S)
OCTINOXATE 7.5%
OXYBENZONE 2%
WARNING
FOR EXTERNAL USE ONLY. NOT TO BE SWALLOWED. AVOID CONTACT WITH EYES. IF CONTACT OCCURS, RINSE EYES THOROUGHLY WITH WATER. DISCONTINUE USE IF SIGNS OF IRRITATION APPEAR. IF IRRITATION PERSISTS. CONSULT A DOCTOR. KEEP OUT OF REACH OF CHILDREN. DO NOT USE ON INFANTS UNDER THE AGE OF SIX MONTHS.
DIRECTIONS FOR USE
SMOOTH ON FACE EACH MORNING.
INACTIVE INGREDIENTS
AQUA (WATER), BUTYLENE GLYCOL, C12-15 ALKYL BENZOATE, GLYCERETH-26, CETYL ALCOHOL, GLYCERYL STEARATE, PROPYLENE GLYCOL, DIMETHICONE, OCTYLDODECYL NEOPENTANOATE, PHENOXYETHANOL, PEG-75 STEARATE, POLYACRYLAMIDE, SACCHARIDE ISOMERATE, JOJOBA ESTERS, PARFUM (FRAGRANCE), CETETH-20, C13-14 ISOPARAFFIN, STEARETH-2, CHLORPHENESIN, HYDROXYPROPYL METHYLCELLULOSE, GLYCERIN, THERMUS THERMOPHILLUS FERMENT, LAURETH-7, CERATONIA SILIQUA (CAROB) FRUIT EXTRACT, SODIUM HYALURONATE, CITRIC ACID, BHT, METHYLPARABEN, POTASSIUM SORBATE, PROPYLPARABEN, ETHYLPARABEN, TOCOPHEROL, BIOTIN [+/-(MAY CONTAIN) CI 77007 (ULTRAMARINES), CI 77491, CI 77492, CI 77499 (IRON OXIDES), CI 77891 (TITANIUM DIOXIDE), MICA]
PRINCIPAL DISPLAY PANEL
HYDRAMAX
ACTIVE
TEINT
ACTIVE MOISTURE
TINTED LOTION
SPF 15
(SHADE)
PRECISION
CHANEL
40 ml 1.35 FL.OZ.
HYDRAMAX ACTIVE TEINTEActive Moisture Tinted Lotion LOTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||