Humco Castor Oil
Humco Castor Oil
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredient
- Purpose
- Use
- Warings
- Ask a doctor before use
- When using this product
- If pregnant or breast feeding
- Keep out of reach of children.
- Directions
- WARNINGS:
- Other Information
- Inactive Ingredient
FULL PRESCRIBING INFORMATION
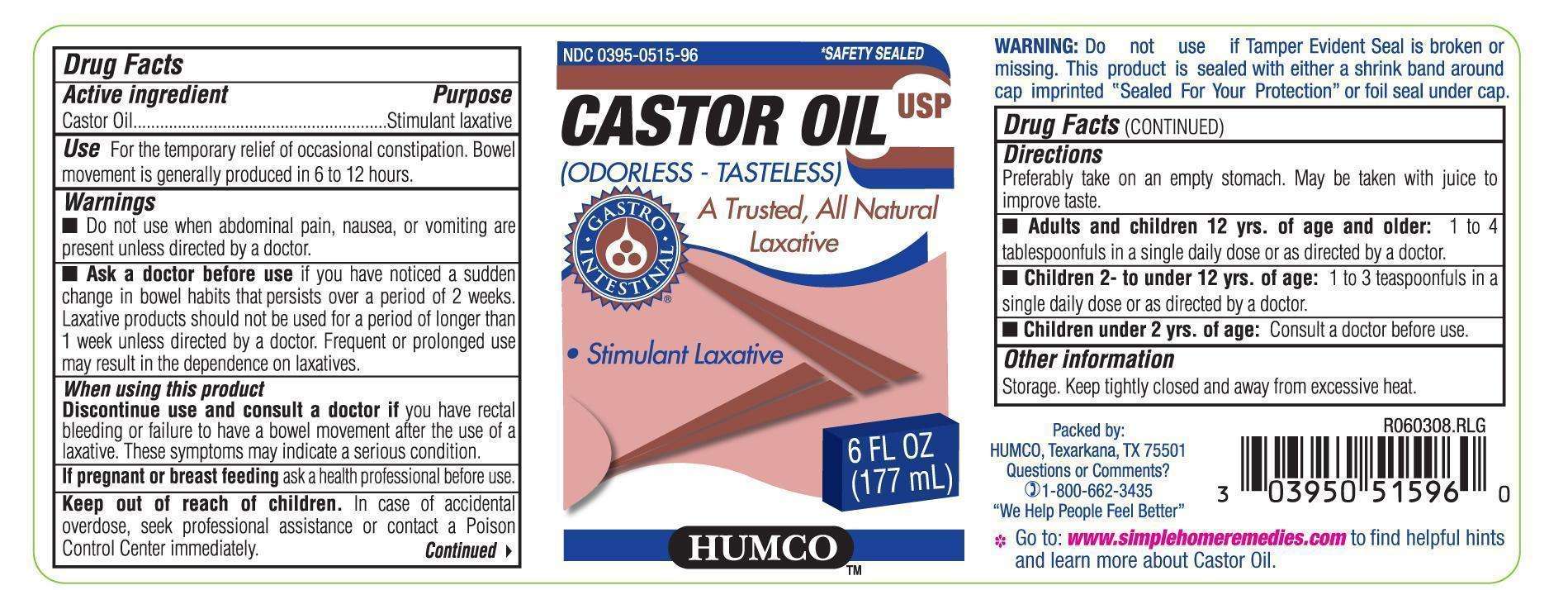
Drug Facts
Active Ingredient
Castor Oil
Purpose
Stimulant Laxative
Use
For the temporary relief of occasional constipation. Bowel movement is generally produced in 6 to 12 hours.
Warings
Do not use when abdominal pain, nausea, or vomiting are present unless directed by a doctor.
Ask a doctor before use
if you have noticed a sudden change in bowel habits that persists over a period of 2 weeks. Laxative products should not be used for a period of longer than 1 week unless directed by a doctor. Frequent or prolonged use may result in the dependence on laxatives.
When using this product
Discontinue use and consult a doctor if you have rectal bleeding or failure to have a bowel movement after the use of a laxative. Thee symptoms may indicate a serious condition.
If pregnant or breast feeding
ask a health professional before use
Keep out of reach of children.
In case of accidental overdose, seek profssional assistance or contact a Poison Control Center immediately.
Directions
Preferably take on an empty stomach. May be taken with juice to improve taste.
Adults and children 12 yrs. of age and older: 1 to 4 tablesponfuls in a single daily dose or as directed by a doctor.
Children 2 to under 12 yrs. of age: 1 to 3 teaspoonfuls in a single dose or as directed by a doctor.
Children under 2 yrs. of age: Consult a doctor before use.
WARNINGS:
Do not use if Taper Evident Seal is broken or missing. This product is sealed with either a shrink band around cap imprinted "Sealed For your Protection" or foil seal under cap.
Other Information
storage. Keep tightly closed and away from excessive heat.
Inactive Ingredient
None
Label 6 oz
Label 16 oz

Humco Castor OilCastor Oil LIQUID
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||