Heparin Sodium
General Injectables & Vaccines, Inc
Heparin Sodium Add-Vantage Vial 10000 un Injection, USP 5mL Single Dose Vial
FULL PRESCRIBING INFORMATION: CONTENTS*
- Description
- Clinical Pharmacology
- Heparin Sodium Indications and Usage
- Contraindications
- Warnings
- Precautions
- Side Effects
- Overdosage
- Dosage and Administration
- How Supplied
- Sample Package Label
FULL PRESCRIBING INFORMATION
Description
Heparin Sodium Injection, USP is a sterile, nonpyrogenic solution of heparin sodium (derived from porcine intestinal mucosa) in water for injection. Each container contains 10000, 12500, 20000 or 25,000 USP Heparin Units; 40 or 80 mg sodium chloride added to render isotonic (see HOW SUPPLIED section for various sizes and strength). May contain sodium hydroxide and/or hydrochloric acid for pH adjustment. pH 6.0 (5.0 to 7.5). The solution contains no bacteriostat, antimicrobial agent or added buffer and is intended for use only as a single-dose injection. When
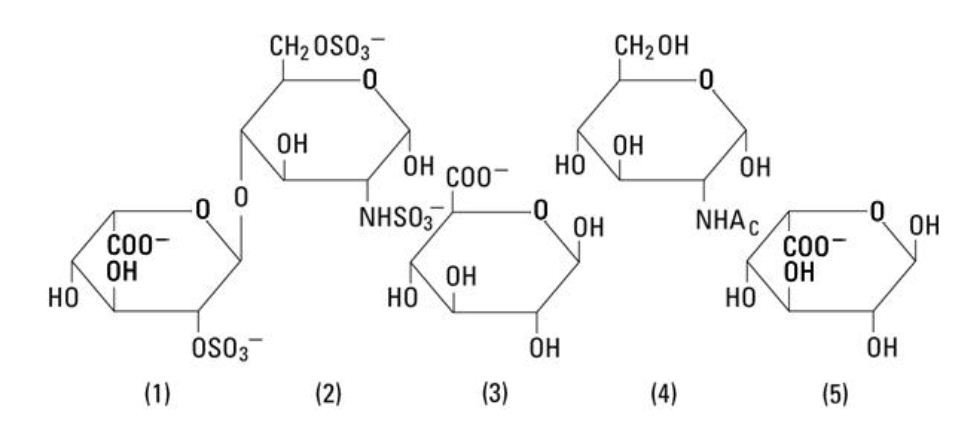
smaller doses are required, the unused portion should be discarded. Heparin sodium in the ADD-Vantage® system is intended for intravenous administration only after dilution. Heparin Sodium, USP is a heterogenous group of straight-chain anionic mucopolysaccharides, called glycosaminoglycans having anticoagulant properties. Although others may be present, the main sugars occurring in heparin are: (1) a- L-iduronic acid 2-sulfate, (2) 2-deoxy-2-sulfamino-a-D-glucose-6-sulfate, (3) b-D-glucuronic acid, (4) 2-acetamido-2-deoxy-a-D-glucose, and (5) a-L-iduronic acid. These sugars are present in decreasing amounts, usually in the order (2) greater than (1)greater than (4) greater than (3) greater than (5), and are joined by glycosidic linkages, forming polymers of varying sizes. Heparin is strongly acidic because of its content of covalently linked sulfate and carboxylic acid groups. In heparin sodium, the acidic protons of the sulfate units are partially replaced by sodium ions. The potency is determined by a biological assay using a USP reference standard based on units of heparin activity per milligram. Structure of Heparin Sodium (representative subunits):
Clinical Pharmacology
Heparin inhibits reactions that lead to the clotting of blood and the formation of fibrin clots both in vitro and in vivo. Heparin acts at multiple sites in the normal coagulation system. Small amounts of heparin in combination with antithrombin III (heparin cofactor) can inhibit thrombosis by inactivating activated Factor X and inhibiting the conversion of prothrombin to thrombin. Once active thrombosis has developed, larger amounts of heparin can inhibit further coagulation by inactivating thrombin and preventing the conversion of fibrinogen to fibrin. Heparin also prevents the formation of a stable fibrin clot in inhibiting the activation of the fibrin stabilizing factor. Bleeding time is usually unaffected by heparin. Clotting time is prolonged by full therapeutic doses of heparin; in most cases, it is not measurably affected by low doses of heparin. Peak plasma levels of heparin are achieved 2 to 4 hours following subcutaneous administration, although there are considerable individual variations. Loglinear plots of heparin plasma concentrations with time for a wide range of dose levels are linear which suggests the absence of zero order processes. Liver and the reticuloendothelial system are the site of biotransformation. The biphasic elimination curve, a rapidly declining alpha phase (t½ = 10#) and after the age of 40 a slower beta phase, indicates uptake in organs. The absence of a relationship between anticoagulant half-life and concentration half-life may reflect factors such as protein binding of heparin.
Heparin does not have fibrinolytic activity; therefore, it will not lyse existing clots.
Heparin Sodium Indications and Usage
Heparin sodium is indicated for:
Atrial fibrillation with embolization:
Diagnosis and treatment of acute and chronic consumption coagulopathies (disseminated intravascular coagulation);
Prevention of clotting in arterial and heart surgery;
Anticoagulant therapy in prophylaxis and treatment of venous thrombosis and its extension;
(In a low-dose regimen) for prevention of postoperative deep venous thrombosis and pulmonary embolism in patients undergoing major abdomino-thoracic surgery or who for other reasons are at risk of developing thromboembolic disease (see DOSAGE AND ADMINISTRATION);
Prophylaxis and treatment of pulmonary embolism;
Prophylaxis and treatment of peripheral arterial embolism.
Contraindications
Heparin sodium should not be used in patients:
With severe thrombocytopenia;
In whom suitable blood coagulation tests — e.g., the whole blood clotting time, partial thromboplastin time, etc. — cannot be performed at appropriate intervals (this contraindication refers to full-dose heparin; there is usually no need to monitor coagulation parameters in patients receiving low-dose heparin);
With an uncontrollable active bleeding state (see WARNINGS), except when this is due to disseminated intravascular coagulation.
Intravenous solutions with Heparin Sodium Injection are contraindicated in patients who are hypersensitive to heparin.
Warnings
Heparin is not intended for intramuscular use.
Hypersensitivity: Patients with documented hypersensitivity to heparin should be given the drug only in clearly life-threatening situations.
Hemorrhage: Hemorrhage can occur at virtually any site in patients receiving heparin. An unexplained fall in hematocrit, fall in blood pressure or any other unexplained symptom should lead to serious consideration of a hemorrhagic event. Heparin sodium should be used with extreme caution in disease states in which there is increased danger of hemorrhage. Some of the conditions in which increased danger of hemorrhage exists are:
Cardiovascular— Subacute bacterial endocarditis. Severe hypertension.
Surgical— During and immediately following (a) spinal tap or spinal anesthesia or (b) major surgery, especially involving the brain, spinal cord or eye.
Hematologic— Conditions associated with increased bleeding tendencies, such as hemophilia, thrombocytopenia, and some vascular purpuras.
Gastrointestinal— Ulcerative lesions and continuous tube drainage of the stomach or small intestine.
Other — Menstruation, liver disease with impaired hemostasis.
Coagulation Testing: When heparin sodium is administered in therapeutic amounts, its dosage should be regulated by frequent blood coagulation tests. If the coagulation test is unduly prolonged or if hemorrhage occurs, heparin sodium should be discontinued promptly (see OVERDOSAGE).
Thrombocytopenia: Thrombocytopenia has been reported to occur in patients receiving heparin with a reported incidence of 0 to 30%. Mild thrombocytopenia (count greater than 100,000/mm3) may remain stable or reverse even if heparin is continued. However, thrombocytopenia of any degree should be monitored closely. If the count falls below 100,000/mm3 or if recurrent thrombosis
develops (see White Clot Syndrome, PRECAUTIONS), the heparin product should be discontinued. If continued heparin therapy is essential, administration of heparin from a different organ source can be reinstituted with caution.
Precautions
General:
Do not administer unless solution is clear and container is intact. Discard unused portion (see DOSAGE AND ADMINISTRATION).
a. White Clot Syndrome:
It has been reported that patients on heparin may develop new thrombus formation in association with thrombocytopenia resulting from irreversible aggregation of platelets induced by heparin, the so-called “white clot syndrome”. The process may lead to severe thromboembolic complications like skin necrosis, gangrene of the extremities that may lead to amputation, myocardial infarction, pulmonary embolism, stroke and possibly death. Therefore, heparin administration should be promptly discontinued if a patient develops new thrombosis in association with thrombocytopenia.
b. Heparin Resistance:
Increased resistance to heparin is frequently encountered in fever, thrombosis, thrombophlebitis, infections with thrombosing tendencies, myocardial infarction, cancer and in postsurgical patients.
c. Increased Risk in Older Women:
A higher incidence of bleeding has been reported in women over 60 years of age.
Laboratory Tests:
Periodic platelet counts, hematocrits and tests for occult blood in stool are recommended during the entire course of heparin therapy (see DOSAGE AND ADMINISTRATION).
Drug Interactions:
Oral anticoagulants: Heparin sodium may prolong the one-stage prothrombin time. Therefore, when heparin sodium is given with dicumarol or warfarin sodium, a period of at least 5 hours after the last intravenous dose should elapse before blood is drawn if a valid PROTHROMBIN time is to be obtained.
Platelet inhibitors: Drugs such as acetylsalicylicacid, dextran, phenylbutazone, ibuprofen, indomethacin, dipyridamole, hydroxychloroquine and others that interfere with platelet aggregation reactions (the main hemostatic defense of heparinized patients) may induce bleeding and should be used with caution in patients receiving heparin sodium.
Other interactions: Digitalis, tetracyclines, nicotine, antihistamines or I.V. nitroglycerin may partially counteract the anticoagulant action of heparin sodium.
Drug/Laboratory Test Interactions:
Hyperaminotransferasemia: Significant elevations of aminotransferase (SGOT [S-AST] and SGPT [S-ALT]) levels have occurred in a high percentage of patients (and healthy subjects) who have received heparin. Since aminotransferase determinations are important in the differential diagnosis of myocardial infarction, liver disease, and pulmonary emboli, rises that might be caused by drugs (like heparin) should be interpreted with caution.
Carcinogenesis, Mutagenesis, Impairment of Fertility:
No long-term studies in animals have been performed to evaluate carcinogenic potential of heparin. Also, no reproduction studies in animals have been performed concerning mutagenesis or impairment of fertility.
Pregnancy:
Teratogenic Effects:Pregnancy Category C. Animal reproduction studies have not been conducted with heparin sodium. It is also not known whether heparin sodium can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Heparin sodium should be given to a pregnant woman only if clearly needed.
Nonteratogenic Effects: Heparin does not cross the placental barrier.
Nursing Mothers:
Heparin is not excreted in human milk.
Pediatric Use:
See DOSAGE AND ADMINISTRATION.
Side Effects
Hemorrhage: Hemorrhage is the chief complication that may result from heparin therapy (see WARNINGS). An overly prolonged clotting time or minor bleeding during therapy can usually be controlled by withdrawing the drug (see OVERDOSAGE). It should be appreciated that gastrointestinal or urinary tract bleeding during anticoagulant therapy may indicate the presence of an underlying occult lesion. Bleeding can occur at any site but certain specific hemorrhagic complications may be difficult to detect:
a. Adrenal hemorrhage, with resultant acute adrenal insufficiency, has occurred during anticoagulant therapy. Therefore, such treatment should be discontinued in patients who develop signs and symptoms of acute adrenal hemorrhage and insufficiency. Initiation of corrective therapy should not depend on laboratory confirmation of the diagnosis since any delay in an acute situation
may result in the patient’s death.
b. Ovarian (corpus luteum) hemorrhage developed in a number of women of reproductive age receiving short- or long-term anticoagulant therapy. This complication if unrecognized may be fatal.
c. Retroperitoneal hemorrhage.
Hypersensitivity: Generalized hypersensitivity reactions have been reported with chills, fever, and urticaria as the most usual manifestations, and asthma, rhinitis, lacrimation, headache, nausea and vomiting and anaphylactoid reactions, including shock, occurring more rarely. Itching and burning, especially on the plantar site of the feet may occur. Thrombocytopenia has been reported to occur in patients receiving heparin with a reported incidence of 0 to 30%. While often mild and of no obvious clinical significance, such thrombocytopenia can be accompanied by severe thromboembolic complications such as skin necrosis, gangrene of the extremities that may lead to amputation, myocardial infarction, pulmonary embolism, stroke and possibly death. (See WARNINGS and PRECAUTIONS.) Certain episodes of painful, ischemic and cyanosed limbs have in the past been attributed to allergic vasospastic reactions. Whether these are in fact identical to the thrombocytopenia associated complications remains to be determined.
Miscellaneous: Osteoporosis following long-term administration of high doses of heparin, cutaneous necrosis after systemic administration, suppression of aldosterone synthesis, delayed transient alopecia, priapism and rebound hyperlipemia on discontinuation of heparin sodium have also been reported. Significant elevations of aminotransferase (SGOT [S-AST] and SGPT [S-ALT]) levels have occurred in a high percentage of patients (and healthy subjects) who have received heparin.
Overdosage
Symptoms: Bleeding is the chief sign of heparin overdosage. Nosebleeds, blood in urine or tarry stools may be noted as the first sign of bleeding. Easy bruising or petechial formations may precede frank bleeding.
Treatment: Neutralization of heparin effect.
When clinical circumstances (bleeding) require reversal of heparinization, protamine sulfate (1% solution) by slow infusion will neutralize heparin sodium. No more than 50 mg should be administered, very slowly in any 10 minute period. Each mg of protamine sulfate neutralizes approximately 100 USP heparin units. The amount of protamine required decreases over time as heparin is metabolized. Although the metabolism of heparin is complex, it may, for the purpose of choosing a protamine dose, be assumed to have a half-life of about ½ hour after intravenous injection. Administration of protamine sulfate can cause severe hypotensive and anaphylactoid reactions. Because fatal reactions often resembling anaphylaxis have been reported, the drug should be given only when resuscitation techniques and treatment of anaphylactoid shock are readily available. For additional information, the labeling of Protamine Sulfate Injection, USP products should be consulted.
Dosage and Administration
Heparin sodium is not effective by oral administration and should be given by intermittent intravenous injection, after dilution in 50 or 100 mL of 5% Dextrose Injection, USP or 0.9% Sodium Chloride Injection, USP, or by intravenous infusion. The dosage of heparin sodium should be adjusted according to the patient’s coagulation test results. When heparin is given by continuous intravenous infusion, the coagulation time should be determined approximately every 4 hours in the early stages of treatment. When the drug is administered intermittently by intravenous injection, coagulation tests should be performed before each injection during the early stages of treatment and at appropriate intervals thereafter. Dosage is considered adequate when the activated partial thromboplastin time (APTT) is 1.5 to 2 times normal or when the whole blood clotting time is elevated approximately 2.5 to 3 times the control value.
Periodic platelet counts, hematocrits and tests for occult blood in stool are recommended during the entire course of heparin therapy.
Converting to Oral Anticoagulant: When an oral anticoagulant of the coumarin or similar type is to be begun in patients already receiving heparin sodium, baseline and subsequent tests of prothrombin activity must be determined at a time when heparin activity is too low to affect the prothrombin time. If continuous I.V. heparin infusion is used, prothrombin time can usually be measured at any time. In converting from heparin to an oral anticoagulant, the dose of the oral anticoagulant should be the usual initial amount and thereafter prothrombin time should be determined at the usual intervals. To ensure continuous anticoagulation, it is advisable to continue full heparin therapy for several days after the prothrombin time has reached the therapeutic range. Heparin therapy may then be discontinued without tapering.
Therapeutic Anticoagulant Effect with Full-Dose Heparin
Although dosage must be adjusted for the individual patient according to the results of suitable laboratory tests, the following dosage schedules may be used as guidelines:
| Method of Administration | Frequencey | Recommended dose* |
| Intermittent Intravenous Injection |
Initial Dose Every 4 to 6 hours |
10,000 Units, in 50-100mL of 5% Dextrose Injection or 0.9% Sodium Chloride Injection 5000-10,000 Units, in 50-100mL of %5 Dextrose Injection or 0.9% Sodium Chloride Injection |
| Continuous Intravenous Injection |
Initial Dose Continuous |
5000 Units by I.V. Injection 20,000-40,000 Units/24 Hours in 5% Dextrose Injection or 0.9% Sodium Chloride Injection |
| Initial Dose: Maintenance Dose: |
50 Units/kg (I.V. drip) 100 Units/kg (I.V. drip) every four hours or 20,000 Units/m(2)/24 hours continuously |
Low-Dose Prophylaxis of Postoperative Thromboembolism
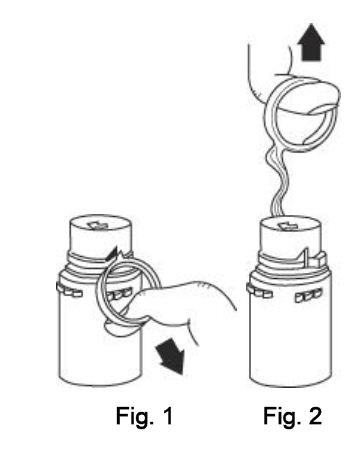
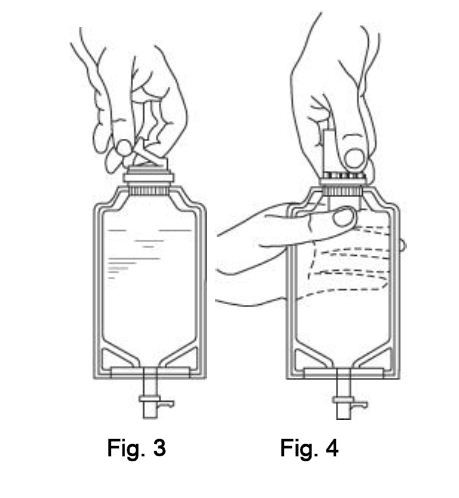
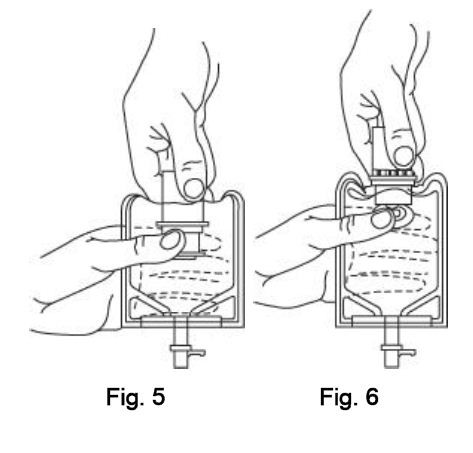
INSTRUCTIONS FOR USE
To Assemble Vial and Flexible Diluent Container:
(Use Aseptic Technique)
How Supplied
| List No. |
Total Volume |
Total Units Heparin/vial |
Total mg Sodium Chloride/vial |
| 2581 |
5 mL |
10,000 |
40 |
| 2582 |
5 mL |
12,500 |
40 |
| 2583 |
10 mL |
20,000 |
80 |
| 2584 |
10 mL |
25,000 |
80 |
Sample Package Label

Heparin SodiumHeparin Sodium INJECTION, SOLUTION, CONCENTRATE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||