Hemorrodil
Hemorrodil Supositorios
FULL PRESCRIBING INFORMATION: CONTENTS*
- Hemorrodil Uses
- Warning
- Directions
- Hemorrodil Other information
- Inactive ingredients
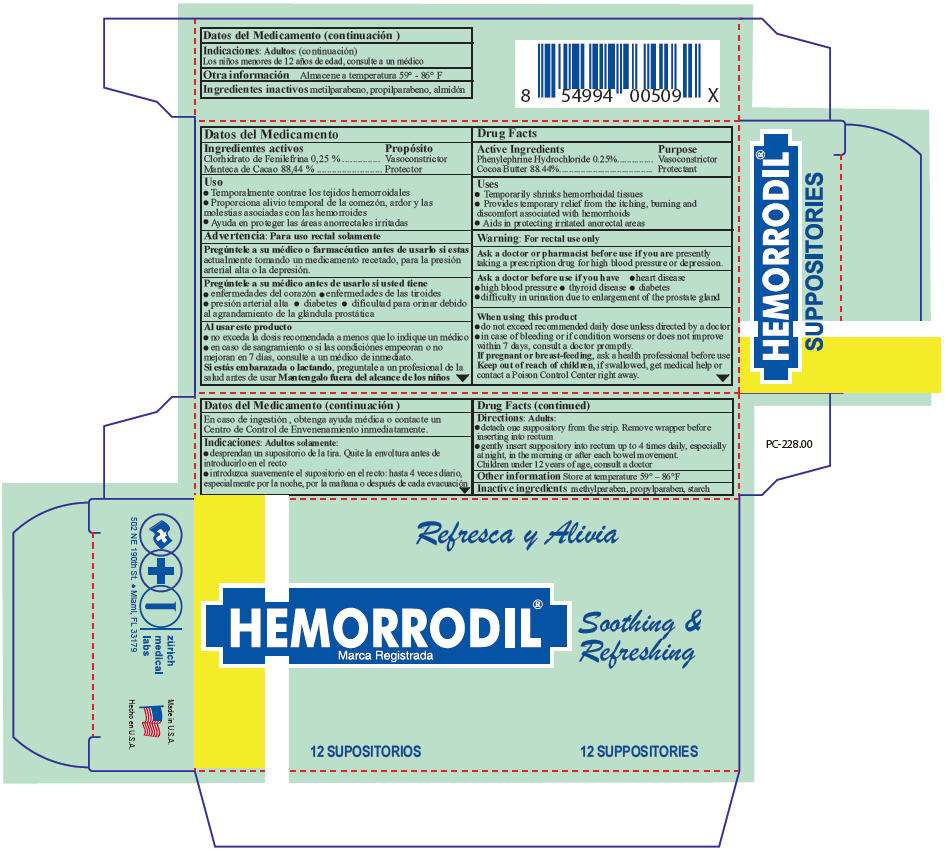
- PRINCIPAL DISPLAY PANEL - 12 Suppository Blister Pack Carton
FULL PRESCRIBING INFORMATION
Drug Facts
Active ingredient
Purpose
| Active Ingredients | Purpose |
|---|---|
| Phenylephrine Hydrochloride 0.25% | Vasoconstrictor |
| Cocoa Butter 88.44% | Protectant |
Hemorrodil Uses
- Temporarily shrinks hemorrhoidal tissues
- Provides temporary relief from the itching, burning and discomfort associated with hemorrhoids
- Aids in protecting irritated anorectal areas
Warning
For rectal use only
Ask a doctor or pharmacist before use if you are presently taking a prescription drug for high blood pressure or depression.
Ask a doctor before use if you have
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- difficulty in urination due to enlargement of the prostate gland
When using this product
- do not exceed recommended daily dose unless directed by a doctor
- in case of bleeding or if condition worsens or does not improve within 7 days, consult a doctor promptly.
If pregnant or breast-feeding, ask a health professional before use
Keep out of reach of children, if swallowed, get medical help or contact a Poison Control Center right away.
Directions
Adults
- detach one suppository from the strip. Remove wrapper before inserting into rectum
- gently insert suppository into rectum up to 4 times daily, especially at night, in the morning or after each bowel movement.
Children under 12 years of age, consult a doctor
Hemorrodil Other information
Store at temperature 59° – 86°F
Inactive ingredients
methylparaben, propylparaben, starch
PRINCIPAL DISPLAY PANEL - 12 Suppository Blister Pack Carton
HEMORRODIL®
Soothing &
Refreshing
12 SUPPOSITORIES
HemorrodilPHENYLEPHRINE HYDROCHLORIDE and COCOA BUTTER SUPPOSITORY
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||