Hemorrhoidal Pad
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient
- Purpose
- Hemorrhoidal Pad Uses
- Warnings
- Directions
- Hemorrhoidal Pad Other information
- Inactive ingredients
- Package label
FULL PRESCRIBING INFORMATION
Active ingredient
Witch hazel 50%
Purpose
Astringent
Hemorrhoidal Pad Uses
- temporarily relieves the local itching and discomfort associated with hemorrhoids
- aids in protecting irritated anorectal areas
- temporarily relieves irritation and burning
Warnings
For external use only.
When using this product
- do not use more than directed unless told to do so by a doctor
- do not put directly in the rectum by using fingers or any mechanical device or applicator
Stop use and ask a doctor if
- rectal bleeding occurs
- condition worsens or does not improve within 7 days
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Adults:
- when practical, clean the affected area with mild soap and warm water, and rinse thoroughly
- gently dry by patting or blotting with toilet tissue or a soft cloth before applying
- apply externally to the affected area up to 6 times daily or after each bowel movement
- after application, discard pad
Hemorrhoidal Pad Other information
- Store at 68° - 77°F (20° - 25°C)
Inactive ingredients
water, glycerin, alcohol, propylene glycol, sodium citrate, diazolidinyl urea, citric acid, methylparaben, propylparaben
Package label
Hemorrhoidal Pads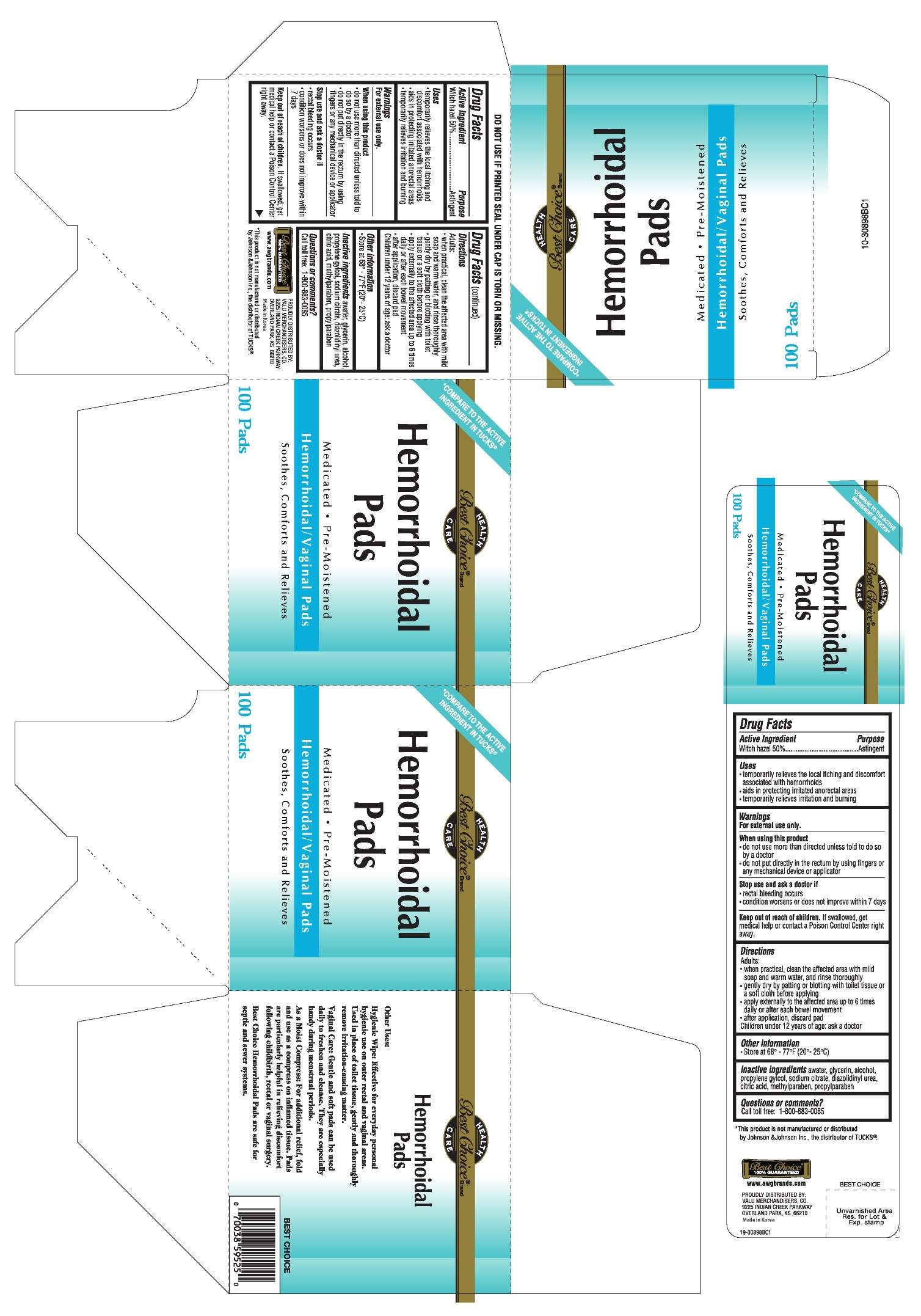
Hemorrhoidal PadWitch Hazel Pad CLOTH
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!