Help I have allergies
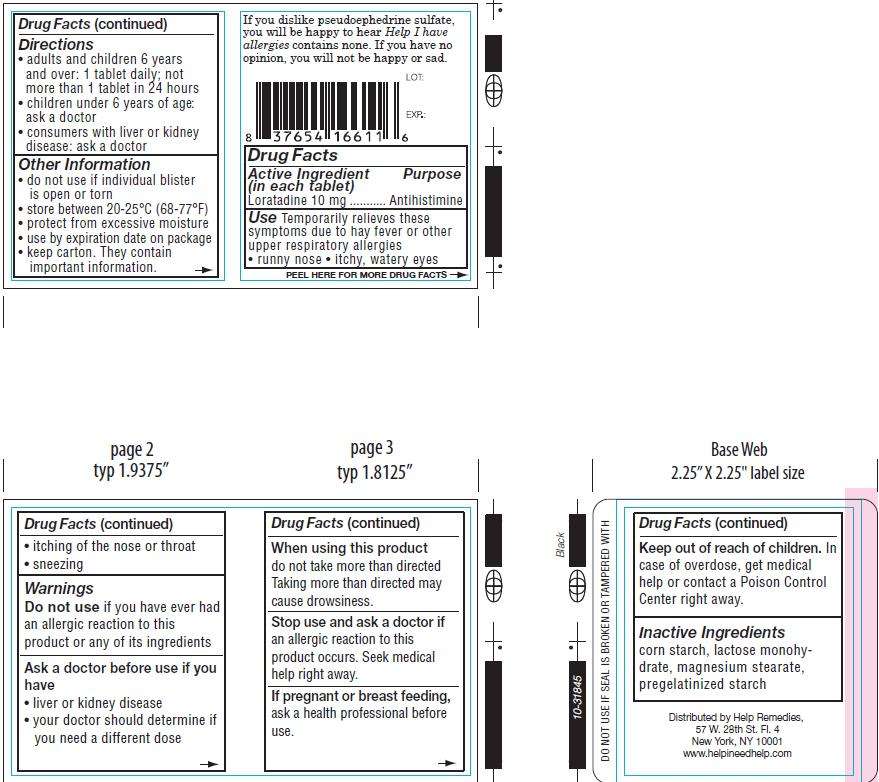
Drug Facts
FULL PRESCRIBING INFORMATION
Active ingredient
Active Ingredient Purpose
(in each tablet)
Loratadine 10mg..........................................Antihistamine
Purpose
Use
- runny nose
- itchy, watery eyes
- itching of the nose or throat
- sneezing
Warnings
Do not use
Ask a doctor before use if you have
- liver or kidney disease
- your doctor should determine if you need a different dose
Stop use and ask a doctor if
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control
Center right away.
If pregnant or breast feeding,
Directions
- adults and children 6 years and over: 1 tablet daily; not more than 1 tablet in 24 hours
- children under 6 years of age: ask a doctor
- consumers with liver or kidney disease: ask a doctor
Uses
Other Information
- do not use if individual blister is open or torn
- store between 20-25 degrees celcius (68-77 degrees fahrenheits)
- protect from excessive moisture
- use by expiration date on package
- keep carton. They contain important information.
Inactive Ingredients
Distributed by Help Remedies,
57 W. 28th St. Fl. 4
New York, NY 10001
www.helpineedhelp.com
Help I have allergiesLoratadine TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!