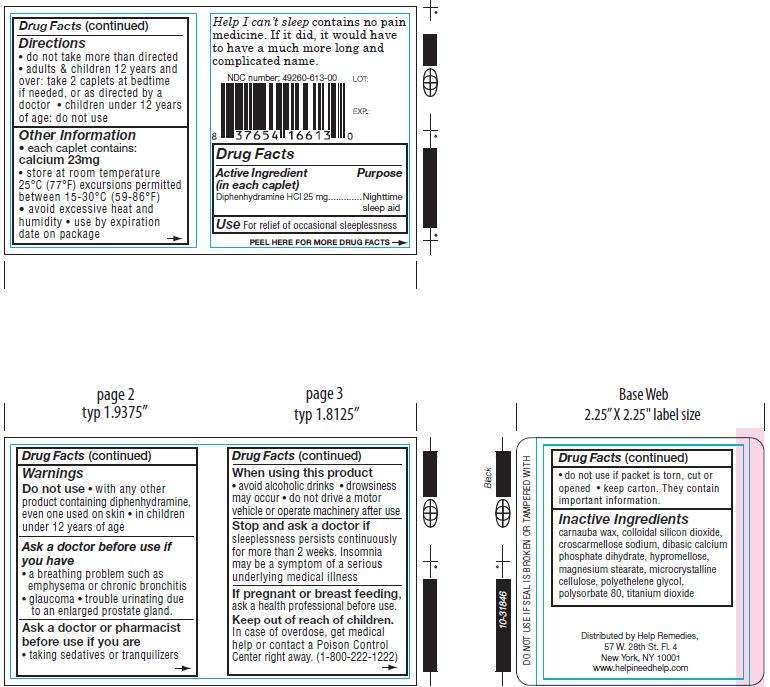
Help I cant Sleep
Help Remedies, Inc.
Help Remedies, Inc.
Drug Facts
FULL PRESCRIBING INFORMATION
Active ingredient
Active Ingredient...................................................Purpose
(in each caplet)
Diphenhydramine HCl 25 mg...................................Nighttime/sleep aid
Purpose
Use: For relief of occasional sleeplessness
Warnings
- Ask a doctor before use if you have • a breathing problem such as emphysema or chronic bronchitis • glaucoma • trouble urinating due to an enlarged prostate gland.
- Ask a doctor or pharmacist before use if you are • taking sedatives or tranquilizers.
- When using this product:
• avoid alcoholic drinks • drowsiness may occur • do not drive a motor vehicle or operate machinery after use.
Do not use
• with any other product containing diphenhydramine, even one used on skin • in children under 12 years of age.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
If pregnant or breast feeding, ask a health professional before use.
Directions
Uses
Other Information
Inactive Ingredients
Distributed by Help Remedies,
57 W. 28th St. Fl. 4
New York, NY 10001
www.helpineedhelp.com
Help I cant SleepDiphenhydramine TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||