Hectorol
FULL PRESCRIBING INFORMATION: CONTENTS*
- HECTOROL DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- HECTOROL CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- HECTOROL ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
HECTOROL DESCRIPTION
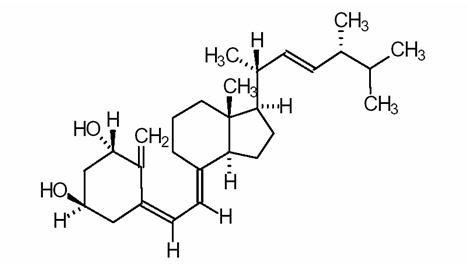
CLINICAL PHARMACOLOGY
Mechanism of Action
Pharmacokinetics and Metabolism
Clinical Studies
Table 1below.
Table 1: iPTH Summary Data for Dialysis Patients Receiving Hectorol
iPTH (pg/mL)
meanss.d.(n *)
p Value v. Baseline
p Value v. Placebo Hectorol Placebo *
Table 2shows the numbers of patients within each group who achieved and maintained iPTH levels below 300 pg/mL during the open-label and double-blind phases. Seventy-four of 138 patients (53.6%) had plasma iPTH levels within the target range (150-300 pg/mL) during Weeks 14-16.
Table 2: Number of Times iPTHpg/mL
12
INDICATIONS & USAGE
Dialysis Patients:Pre-Dialysis Patients:
HECTOROL CONTRAINDICATIONS
WARNINGS
OVERDOSAGE). Progressive hypercalcemia due to overdosage of vitamin D and its metabolites may be so severe as to require emergency attention. Acute hypercalcemia may exacerbate tendencies for cardiac arrhythmias and seizures and may potentiate the action of digitalis drugs. Chronic hypercalcemia can lead to generalized vascular calcification and other soft-tissue calcification. The serum calcium times serum phosphorus (Ca X P) product should be maintained at < 55 mg2/dL2 in patients with chronic kidney disease. Radiographic evaluation of suspect anatomical regions may be useful in the early detection of this condition.DOSAGE AND ADMINISTRATIONsection.)
PRECAUTIONS
GeneralInformation for the Patient
ADVERSE REACTIONSsection).
Laboratory Tests
Drug Interactions
(see WARNINGS). The use of mineral oil or other substances that may affect absorption of fat may influence the absorption and availability of Hectorol. Although not examined specifically, enzyme inducers (such as glutethimide and phenobarbital) may affect the 25-hydroxylation of Hectorol and may necessitate dosage adjustments. Cytochrome P450 inhibitors (such as ketoconazole and erythromycin) may inhibit the 25-hydroxylation of Hectorol. Hence, formation of the active Hectorol moiety may be hindered.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Use in Pregnancy
Nursing Mothers
Pediatric Use
Geriatric Use
Hepatic Insufficiency
HECTOROL ADVERSE REACTIONS
Dialysis:CLINICAL PHARMACOLOGY/Clinical Studies) and in 3.3% of 61 patients treated with placebo for two months. Adverse events occurring in the Hectorol group at a frequency of 2% or greater and more frequently than in the placebo group are presented inTable 3below:
Table 3: Adverse Events Reported by2% of HectorolTreated Patients and More Frequently Than Placebo During the Double-blind Phase of Two Clinical Studies
Adverse Event Hectorol(n=61)
% Placebo (n=61)
% Body as a Whole Cardiovascular System Digestive System Musculoskeletal System Metabolic and Nutritional Nervous System Respiratory System Skin
Pre-dialysis:
CLINICAL PHARMACOLOGY/Clinical Studies) and in three (10.7%) of 28 patients treated with placebo for 24 weeks. Adverse events occurring in the Hectorol group at a frequency of 5% or greater and more frequently than in the placebo group are as follows:Body as a WholeDigestive SystemHematologic and LymphaticMetabolic and NutritionalNervous SystemRespiratory SystemCough increased, Dyspnea, Rhinitis.
OVERDOSAGE
Treatment of Hypercalcemia and Overdosage
General treatment of hypercalcemia (greater than 1 mg/dL above the upper limit of the normal range in dialysis patients; greater than 10.7 mg/dL in pre-dialysis patients) consists of immediate suspension of Hectorol therapy, institution of a low calcium diet, and withdrawal of calcium supplements. Serum calcium levels should be determined at least weekly until normocalcemia ensues. Hypercalcemia usualy resolves in 2 to 7 days. When serum calcium levels have returned to within normal limits, Hectorol therapy may be reinstituted at a dose that is lower (at least 2.5 mcg in dialysis patients and 0.5 mcg in pre-dialysis patients) than prior therapy. In dialysis patients, serum calcium levels should be obtained weekly after all dosage changes and during subsequent dosage titration. Persistent or markedly elevated serum calcium levels may be corrected by dialysis against a reduced calcium or calcium-free dialysate.
Treatment of Accidental Overdosage of Doxercalciferol
DOSAGE & ADMINISTRATION
Adult Administration:Table 4provides the current recommended therapeutic target levels for iPTH in patients with chronic kidney disease:
Table 4: Target Range of Intact Plasma PTH by Stage of CKD
CKD Stage
GFR
(mL/min/1.73m2) TargetPTH
(pg/mL)
Dialysis:
Table 5: Dialysis Dosing Recommendations
Initial DosingiPTH Level HectorolDose
Dose TitrationiPTH Level HectorolDose
Pre-dialysis:
Table 6presents a suggested approach in dose titration:
Table 6: Pre-dialysis Dosing Recommendations
Initial DosingiPTH Level HectorolDose
Dose TitrationiPTH Level HectorolDose
HOW SUPPLIED
STORAGE AND HANDLING
Store at 25 C (77 F) excursions permitted to 15-30 c(59-86 F)[see USP controlled room temperature]
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Hectoroldoxercalciferol CAPSULE, LIQUID FILLED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!