Head and Shoulders Conditioner
Procter & Gamble Manufacturing Co.
Head and ShouldersConditioner Dry Scalp
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient
- Purpose
- Head and Shoulders Conditioner Uses
- Warnings
- Directions
- Inactive ingredients
- Questions (or comments)?
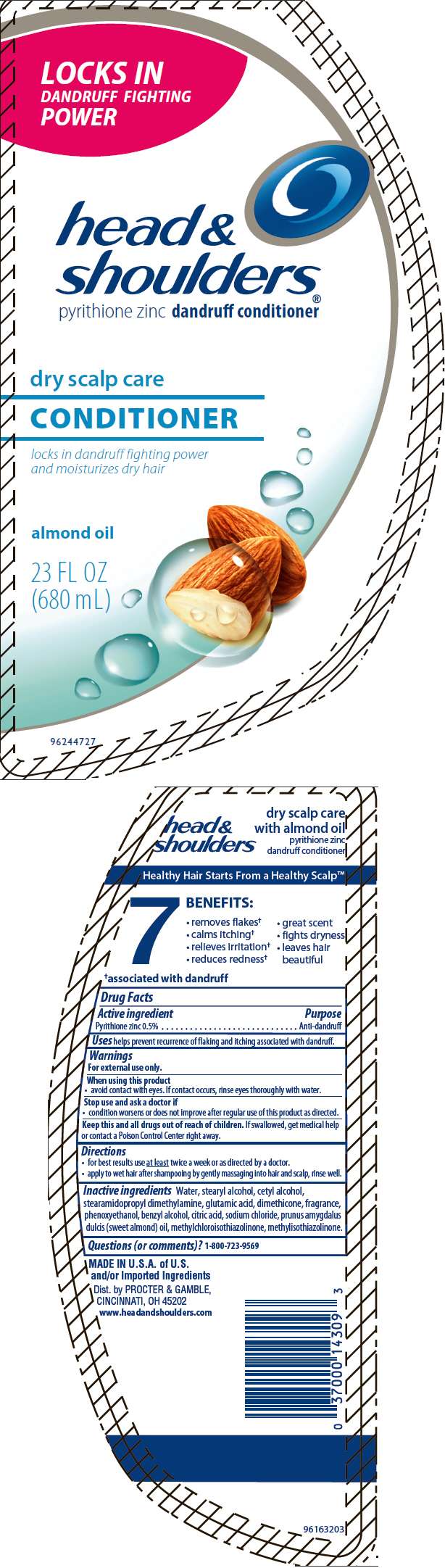
- PRINCIPAL DISPLAY PANEL - 680 mL Bottle Label
FULL PRESCRIBING INFORMATION
Drug Facts
Active ingredient
Pyrithione zinc 0.5%
Purpose
Anti-dandruff
Head and Shoulders Conditioner Uses
helps prevent recurrence of flaking and itching associated with dandruff.
Warnings
For external use only.
When using this product
- avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water.
Stop use and ask a doctor if
- condition worsens or does not improve after regular use of this product as directed.
Keep this and all drugs out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- for best results use at least twice a week or as directed by a doctor.
- apply to wet hair after shampooing by gently massaging into hair and scalp, rinse well.
Inactive ingredients
Water, stearyl alcohol, cetyl alcohol, stearamidopropyl dimethylamine, glutamic acid, dimethicone, fragrance, phenoxyethanol, benzyl alcohol, citric acid, sodium chloride, prunus amygdalus dulcis (sweet almond) oil, methylchloroisothiazolinone, methylisothiazolinone.
Questions (or comments)?
1-800-723-9569
Dist. by PROCTER & GAMBLE,
CINCINNATI, OH 45202
PRINCIPAL DISPLAY PANEL - 680 mL Bottle Label
LOCKS IN
DANDRUFF FIGHTING
POWER
head &
shoulders®
pyrithione zinc dandruff conditioner
dry scalp care
CONDITIONER
locks in dandruff fighting power
and moisturizes dry hair
almond oil
23 FL OZ
(680 mL)
96244727
Head and Shoulders ConditionerPyrithione Zinc LOTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||