Hand Gel Prolim Instant Hand Sanitizer
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredient
- Purpose
- Hand Gel Prolim Instant Hand Sanitizer Uses
- Warnings
- When using this product
- Keep out of reach of children
- Directions
- Inactive Ingredients
- Questions?
- Principal Display Panel
FULL PRESCRIBING INFORMATION
Active Ingredient
Ethyl Alcohol 70%
Purpose
Antiseptic
Hand Gel Prolim Instant Hand Sanitizer Uses
- Recommended for aseptic hand cleansing. This product does not replace the use of soap and water.
- Recommended for repeated use.
Warnings
For external use only
When using this product
- Keep out of eyes. In case of accidental contact, flush eyes thoroughly with water for 15 minutes.
- Avoid contact with broken skin or you have allergies to any ingredients.
Stop use and consult a doctor if skin irritation occurs.
Keep out of reach of children
- In case of accidental ingestion drink plenty of water and seek medical help or contact a Poison Controll Center immediately.
- External use only.
FLAMMABLE: Keep out of heat.
Directions
- Place a small amount of product in your hand.
- Rub hands together briskly for at least 30 seconds until dry.
- Children under 6 years of age should use under adult supervision.
Inactive Ingredients
Water, Isopropyl Alcohol, Isopropyl Myristate, Propylene Glycol, Carbomer, DDM Hydantoin, Aminomethyl Propanol, Fragrance.
Questions?
555-555-5555
Principal Display Panel
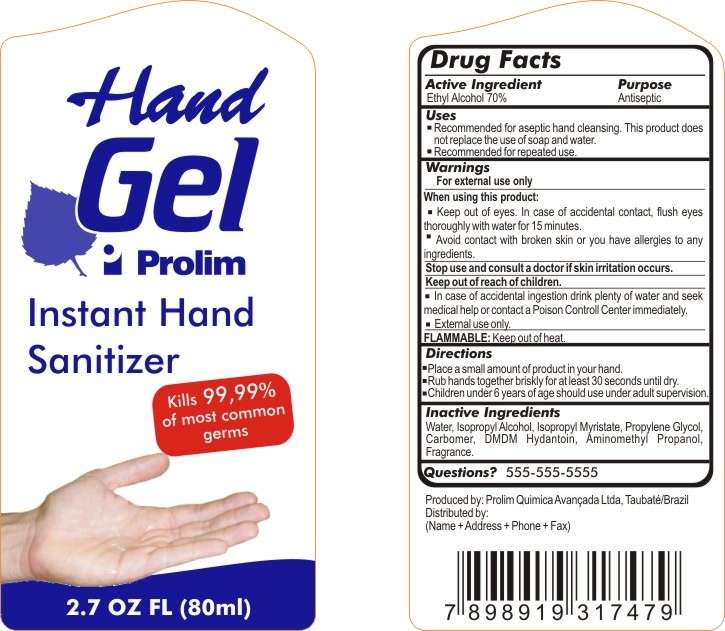
HAND GEL PROLIM
INSTANT HAND SANITIZER
Kills 99,99% of most commom germs
2.7 OZ FL (80ml)
Hand Gel Prolim Instant Hand SanitizerAlcohol GEL
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!