Halonate
Halonate (halobetasol Propionate ointmanet 0.05%)
FULL PRESCRIBING INFORMATION: CONTENTS*
- Description
- Clinical Pharmacology
- Pharmacokinetics
- Halonate Indications and Usage
- Contraindications
- Precautions
- Information for Patients
- Laboratory Tests
- Carcinogenesis, Mutagenesis and Impairment of Fertility
- Pregnancy
- Nursing Mothers
- Pediatric Use
- Geriatric Use
- Adverse Reaction
- Overdosage
- Dosage and Adminstration
- How Supplied
FULL PRESCRIBING INFORMATION
Description
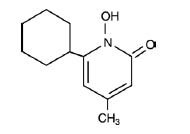
Halonate Halobetasol Propionate Ointment, 0.05% contains halobetasol propionate, a synthetic corticosteroid for topical dermatological use. The corticosteroids constitute a class of primarily synthetic steroids used topically as an anti-inflammatory and anti-pruritic agent. Chemically halobetasol propionate is 21-chloro-6α, 9-difluoro-11β,17-dihydroxy-16β-methylpregna-1, 4-diene-3-20-dione, 17-propionate, C25H31ClF2O5. It has the following structural formula:
Clinical Pharmacology
Pharmacokinetics
Halonate Indications and Usage
Contraindications
Halobetasol Propionate Ointment is contraindicated in those patients with a history of hypersensitivity to any of the components of the preparation.
Precautions
Pediatric Use
Information for Patients
Laboratory Tests
Carcinogenesis, Mutagenesis and Impairment of Fertility
Long-term animal studies have not been performed to evaluate the carcinogenic potential of halobetasol propionate. Positive mutagenicity effects were observed in two genotoxicity assays. Halobetasol propionate was positive in a Chinese hamster micronucleus test, and in a mouse lymphoma gene mutationassay in vitro. Studies in the rat following oral administration at dose levels up to 50 μg/kg/day indicated no impairment of fertility or general reproductive performance. In other genotoxicity testing, halobetasol propionate was not found to be genotoxic in the Ames/Salmonella assay, in the sister chromatid exchange test in somatic cells of the Chinese hamster, in chromosome aberration studies of germinal and somatic cells of rodents, and in a mammalian spot test to determine point mutations.
Pregnancy
Teratogenic effects: Pregnancy Category C:Nursing Mothers
Systemically administered corticosteroids appear in human milk and could suppress growth, interfere with endogenous corticosteroid production, or cause other untoward effects. It is not known whether topical administration of corticosteroids could result in sufficient systemic absorption to produce detectable quantities in human milk. Because many drugs are excreted in human milk,caution should be exercised when Halobetasol Propionate Ointment is administered to a nursing woman.
Pediatric Use
Safety and effectiveness of Halobetasol Propionate Ointment in pediatric patients have not been established and use in pediatric patients under 12 is not recommended. Because of a higher ratio of skin surface area to body mass, pediatric patients are at a greater risk than adults of HPA axis suppression and Cushing’s syndrome when they are treated with topical corticosteroids. They are therefore also at greater risk of adrenal insufficiency during or after withdrawal of treatment. Adverse effects including striae have been reported with inappropriate use of topical corticosteroids in infants and children. HPA axis suppression, Cushing’s syndrome, linear growth retardation, delayed weight gain and intracranial hypertension have been reported in children receiving topical corticosteroids. Manifestations of adrenal suppression in children include low plasma cortisol levels and an absence of response to ACTH stimulation. Manifestations of intracranial hypertension include bulging fontanelles, headaches, and bilateral papilledema.
Geriatric Use
Adverse Reaction
Overdosage
PRECAUTIONS
Dosage and Adminstration
How Supplied
1-800-499-4468
www.innocutis.com


HalonateHalobetasol OINTMENT
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||