GUNA-MATRIX
DRUG FACTS
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
ACTIVE INGREDIENTS/PURPOSE
ASCORBIC ACID 4X ANTIOXIDANT
CONJUNCTIVAL TISSUE 6X DETOXIFICATION
DEHYDROEPIANDROSTERON 6X HORMONAL SUPPORT
DL-MALIC ACID 6X PROMOTE CELL METABOLISM
FUCUS VESICULOSUS 4X DETOXIFICATION
HISTIDINE 4X ANTI-INFLAMMATORY
HYALURONIDASE 6X ANTI-INFLAMMATORY
INTERLEUKIN 6 4C IMMUNE SUPPORT
LACTICUM ACIDUM 4X ANTIOXIDANT
LYMPHATIC VESSEL 6X IMMUNE SUPPORT
NADIDUM 6X METABOLIC SUPPORT
NATRUM OXALACETICUM 6X ANTIOXIDANT
NATRUM PYRUVICUM 6X ANTIOXIDANT
NATRUM SULPHURICUM 6X, 8X, 12X, 30X, 200X ANTIOXIDANT
PHENYLALANINE 4X IMPROVE CONCENTRATION
PROLACTIN 6X IMMUNE SUPPORT
PYROGENIUM 12X ANTI-INFLAMMATORY
THUJA OCCIDENTALIS 6X, 8X, 12X, 30X, 200X DETOXIFICATION
TRICHINOYL PHOSPHATE 6X ANTIOXIDANT
TYROSINE 4X IMMUNE SUPPORT
USES
For Temporary Relief of Symptoms due to Inflammation such as: Loss of Energy, Poor Concentration, Minor Aches and Pains
WARNINGS
PREGNANCY
If pregnant or breast-feeding ask a doctor before use
WARNINGS
Keep this and all medicines out of reach of children
DIRECTIONS
Take 15 minutes before meals
Adults and children 12 years and older 20 drops in a little water 2 times per day
Children between 12 years and 6 years of age 10 drops in a little water 2 times per day
Children under 6 years 5 drops in a little water 2 times per day
QUESTIONS
Questions?: info@gunainc.com
Tel. (484) 223-3500
PRINCIPAL DISPLAY PANEL
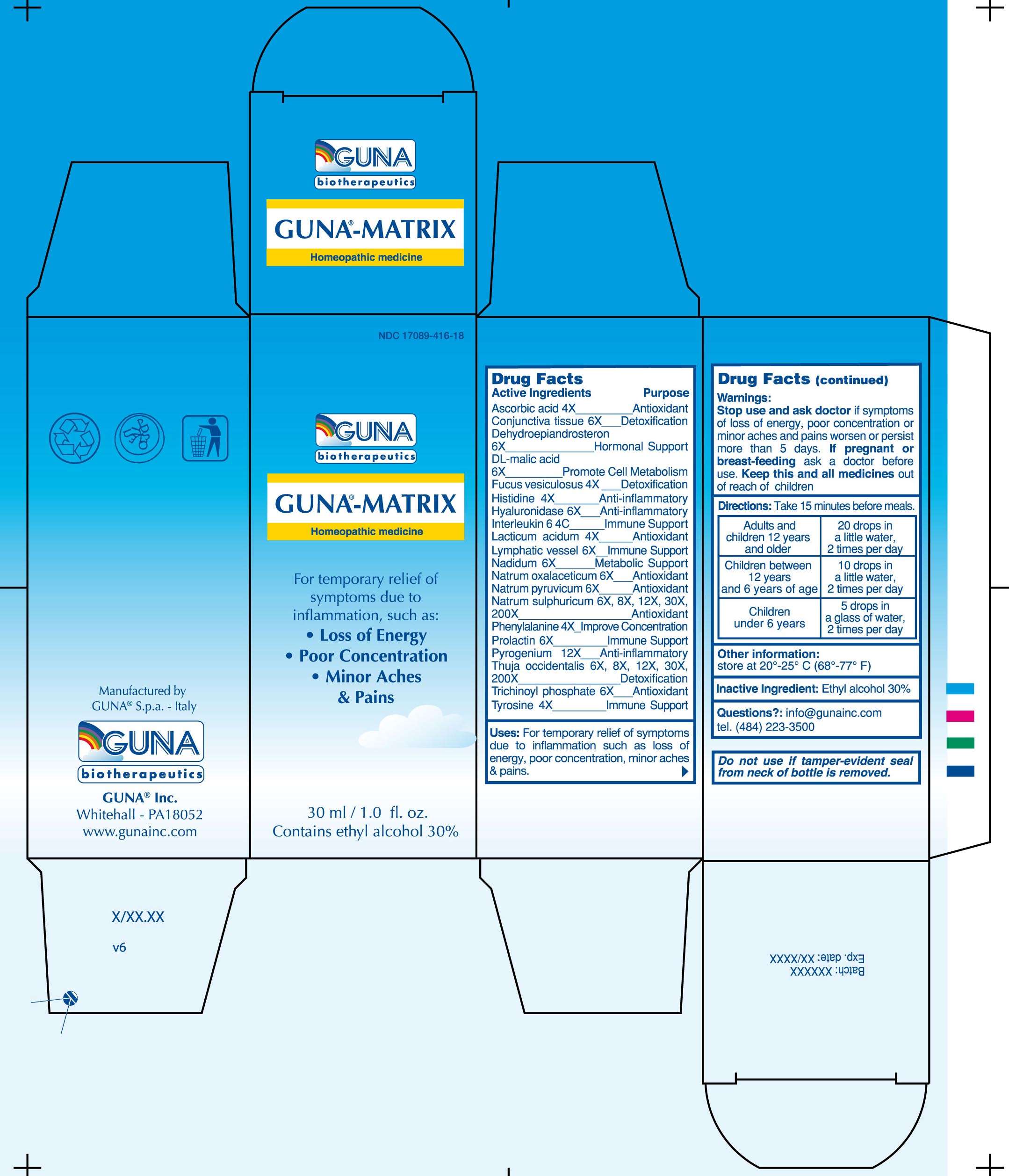
GUNA-MATRIXASCORBIC ACID - DODECAHYDROXYCYCLOHEXANE DIHYDRATE - FUCUS VESICULOSUS - HISTIDINE MONOHYDROCHLORIDE - HUMAN INTERLEUKIN-6 (NONGLYCOSYLATED) - HYALURONIDASE - LACTIC ACID, DL - MALIC ACID - NADIDE - PHENYLALANINE - PRASTERONE - PROLACTIN - RANCID BEEF - SODIUM DIETHYL OXALACETATE - SODIUM PYRUVATE - SODIUM SULFATE - SUS SCROFA CONJUNCTIVA - SUS SCROFA SMALL INTESTINE MUCOSA LYMPH FOLLICLE - THUJA OCCIDENTALIS TWIG - TYROSINE - SOLUTION/ DROPS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||