GUNA-DIUR
GUNA®-DIUR
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1. GUNA-DIUR INDICATIONS AND USAGE
- 2. GUNA-DIUR DOSAGE AND ADMINISTRATION
- 3. DOSAGE FORMS AND STRENGTHS
- 4. GUNA-DIUR CONTRAINDICATIONS
- 5. WARNINGS AND PRECAUTIONS
- 6. GUNA-DIUR ADVERSE REACTIONS
- 7. DRUG INTERACTIONS
- 8. USE IN SPECIFIC POPULATIONS
- 9. DRUG ABUSE AND DEPENDENCE
- 10. OVERDOSAGE
- 11. GUNA-DIUR DESCRIPTION
- 12. CLINICAL PHARMACOLOGY
- 13. NONCLINICAL TOXICOLOGY
- 14. CLINICAL STUDIES
- 15. REFERENCES
- 16. HOW SUPPLIED/STORAGE AND HANDLING
- 17. PATIENT COUNSELING INFORMATION
- PACKAGE LABEL
FULL PRESCRIBING INFORMATION
1. INDICATIONS AND USAGE
1.1. Essential Hypertension (use with GUNA-MALE/FEM)
1.2. Fluid retention (use with GUNA-PMS)
1.3. Temporary weight gain (use with GUNA-MATRIX and GUNA-LYMPHO)
1.4. Lymphedema (use with GUNA-LYMPHO)
1.5. Tissue swelling
Administration may vary according to individual needs.
GUNA®-DIUR may be used together with other homeopathic medications
2. DOSAGE AND ADMINISTRATION
The usual initial dose of GUNA®-DIUR is:
Adults and children 12 years and older 20 drops in a little water, 2 times per day
Children between 12 years and 6 years of age 10 drops in a little water, 2 times per day
Children under 6 years 5 drops in a glass of water, 2 times per day
For an average of two months. If response is not satisfactory, add other products as suggested by your health care professional.
3. DOSAGE FORMS AND STRENGTHS
3.1. 30 ml Bottle dropper.
3.2. Each ingredient is attenuated according to the procedures stated in the Homeopathic Pharmacopeia of the United States. Amiloride 4x, Apis Mellifica 2x, Berberis Vulgaris T, Hydrochlorothiazide 4x, Hypophysis 12x, Mouse-Ear Hawkweed T, Solidago Virgaurea T, Spironolactone 4x.
Inactive Ingredient: Ethylic Alcohol 30%
4. CONTRAINDICATIONS
4.1. There is no history of hypersensitivity to GUNA®-DIUR. However patients with a known hypersensitivity to any GUNA®-DIUR ingredient should consult a physician prior to taking the medication
5. WARNINGS AND PRECAUTIONS
5.1. GUNA®-DIUR is contraindicated in patients with anuria and in patients with a history of hypersensitivity to Spironolactone, Amiloride, or Hydrocholorthiazide.
5.2. Use with caution in patients taking diuretic medications.
6. ADVERSE REACTIONS
6.1. None known (see CONTRAINDICATIONS for hypersensitivity information).
7. DRUG INTERACTIONS
7.1. None Known
8. USE IN SPECIFIC POPULATIONS
8.1. Pregnancy: Pregnancy category C. Animal reproduction studies have not been conducted with GUNA®-DIUR. GUNA®- DIUR should not be given to a pregnant woman.
8.2. Nursing mothers: It is not known whether any of the ingredients in GUNA®- DIUR are secreted in human milk. However, since many drugs are secreted in human milk, caution should be exercised when GUNA®- DIUR is administered to a nursing woman.
8.3. Pediatric use: See DOSAGE AND AMINISTRATION.
8.4. Geriatric use: No restrictions.
9. DRUG ABUSE AND DEPENDENCE
9.1. No Known.
10. OVERDOSAGE
10.1. No Known.
11. DESCRIPTION
GUNA-DIUR is a biochemical homeopathic medication indicated for the treatment of fluid retention, swelling, essential hypertension, lymphedema, and related discomforts.
12. CLINICAL PHARMACOLOGY
12.1. Mechanism of Action
The active ingredients in GUNA®- DIUR are simple biochemical compounds. The exact mechanism of action is unknown; however, due to the homeopathic nature of the active ingredients, receptors may be activated by feedback regulation.
12.2. Pharmacodynamics
The physiological effects of GUNA®-DIUR are due to the action of the ingredients, as described in the Homeopathic Materia Medica.
In Homeopathy there is no direct relationship between dose and effect, but rather there is a relationship between attenuation and balancing effect on biochemical pathways.
In GUNA®-DIUR the attenuation of each ingredient has been selected according to Arndt-Schulz Principle (inverted effect law). The attenuation of the physiological ingredients promotes membrane receptor feedback in order to normalize altered biological pathways. In Addition the attenuation technique activates the low dilutions and stabilizes clinical activity of the compound.
12.3. Pharmacokinetics
The homeopathic attenuation provides complete bioavailability of the active ingredients.
13. NONCLINICAL TOXICOLOGY
13.1. GUNA®-DIUR has no level of toxicity due to the attenuation of the ingredients
14. CLINICAL STUDIES
14.1. GUNA®-DIUR formulation is based on classical Homeopathy and each ingredient has been selected according to its description in the Homeopathic Materia Medica
15. REFERENCES
15.1. H.H. Reckeweg. Homeopathic Materia Medica. Aurelia Verlag.
15.2. Boericke, William, Materia Medica with Reperatory, 1927, ninth edition
16. HOW SUPPLIED/STORAGE AND HANDLING
17. PATIENT COUNSELING INFORMATION
17.1. Patients should be informed about Homeopathy and the main differences with conventional clinical approaches.
PACKAGE LABEL
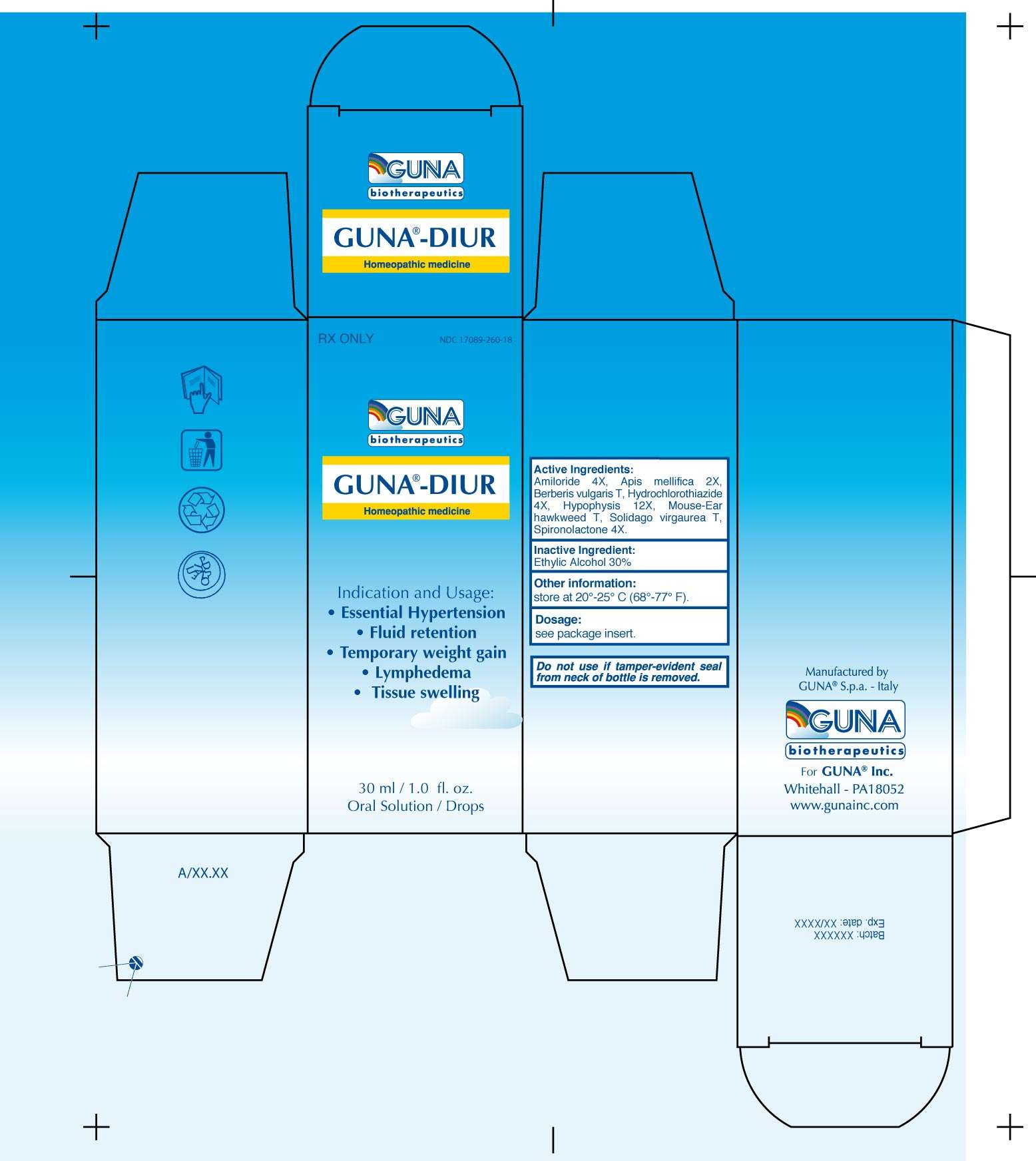
GUNA-DIURAMILORIDE - APIS MELLIFERA - BERBERIS VULGARIS FRUIT - HIERACIUM PILOSELLA FLOWERING TOP - HYDROCHLOROTHIAZIDE - SOLIDAGO VIRGAUREA FLOWERING TOP - SPIRONOLACTONE - SUS SCROFA PITUITARY GLAND - SOLUTION/ DROPS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||