GUNA-COUGH
DRUG FACTS
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
ACTIVE INGREDIENTS/PURPOSE
ALTHAEA OFFICINALIS 1X EXPECTORANT
ANTIMONIUM TARTARICUM 8X ANTITUSSIVE, DRY COUGH
BRYONIA ALBA 6X ANTI-INFLAMMATORY
CETRARIA ISLANDICA 4X EXPECTORANT
COCCUS CACTI 6X ANTITUSSIVE, WET COUGH
CUPRUM ACETICUM 8X PAIN RELIEF
DROSERA 1X COUGH SUPPRESSANT, SPASMODIC COUGH
ECHINACEA ANGUSTIFOLIA 1X IMMUNE SUPPORT
PLANTAGO MAJOR 1X EXPECTORANT
STICTA PULMONARIA 4X ANTITUSSIVE
THYME 1X EXPECTORANT
USES
For the temporary relief of coughs due to minor throat and bronchial irritation from inhaled irritants. Helps loosen phlegm (mucous) and thin bronchial secretions to make coughs more productive
WARNINGS
Ask a doctor before use if you have persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema, or a cough accompanied by too much phlegm (mucus). Stop use and ask a doctor if cough lasts more than 7 days, comes back, or occurs with fever, rash, or persistent headache. These could be signs of a serious illness.
PREGNANCY
If pregnant or breast-feeding ask a doctor before use
WARNINGS
Keep this and all medicines out of reach of children
DIRECTIONS
Use enclosed dosage cup for dosing
Adults and children 12 years and older 10 ml every 4 hours as needed
Children between 12 years and 2 years of age 5 ml every 4 hours as needed
Children under 2 years Ask a physician
QUESTIONS
Questions?: info@gunainc.com
Tel. (484) 223-3500
PRINCIPAL DISPLAY PANEL
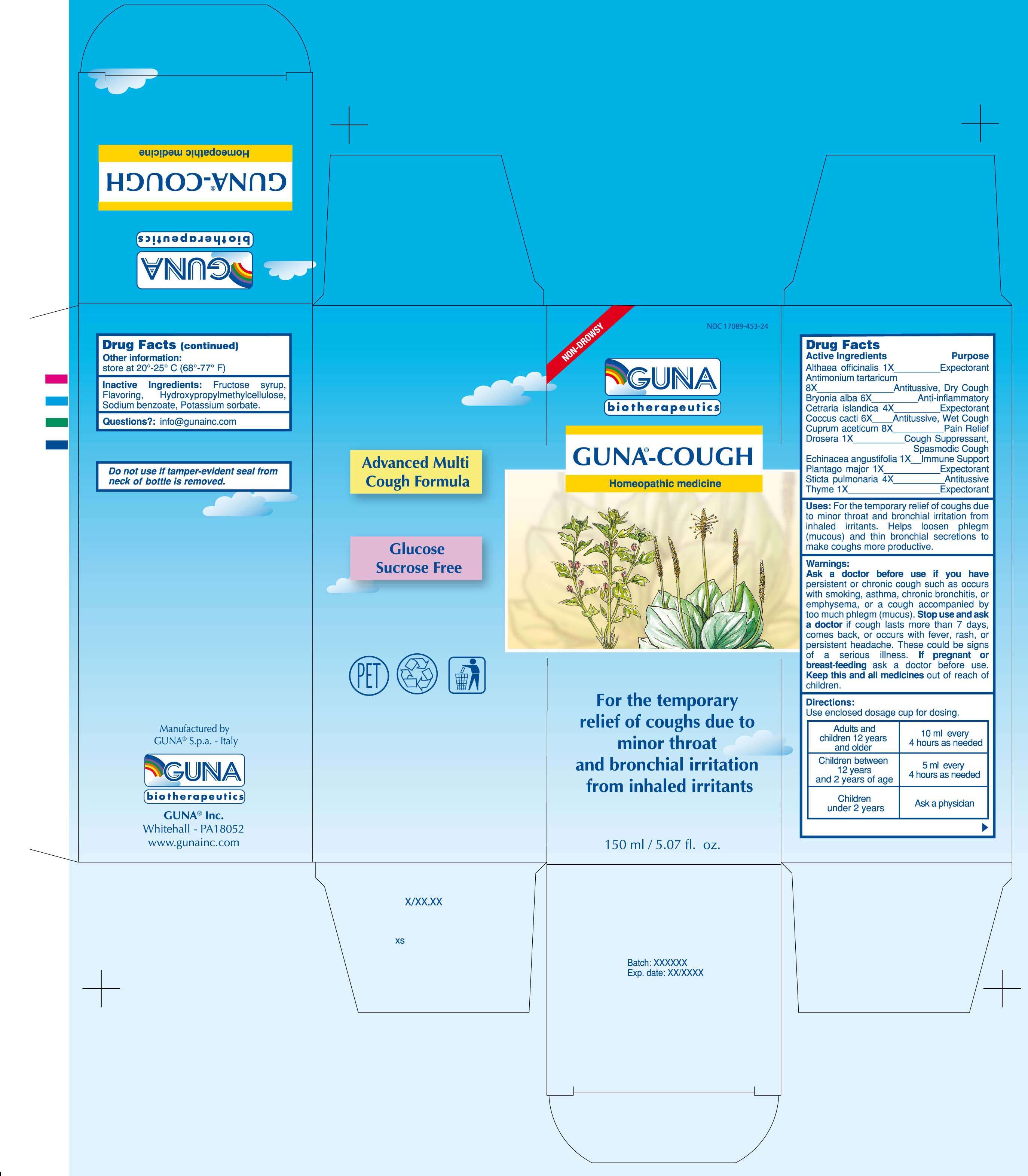
GUNA-COUGHALTHAEA OFFICINALIS LEAF - ANTIMONY POTASSIUM TARTRATE - BRYONIA ALBA ROOT - CETRARIA ISLANDICA SUBSP. ISLANDICA - COCHINEAL - COPPER - DROSERA ROTUNDIFOLIA - ECHINACEA ANGUSTIFOLIA - GARDEN THYME - LOBARIA PULMONARIA - PLANTAGO MAJOR - SOLUTION/ DROPS
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||