Good Care Povidone Iodine Swabsticks
Dalian Goodwood Medical Care Ltd.
Dalian Goodwood Medical Care Ltd.
Good Care Povidone Iodine, USP Swabsticks
FULL PRESCRIBING INFORMATION: CONTENTS*
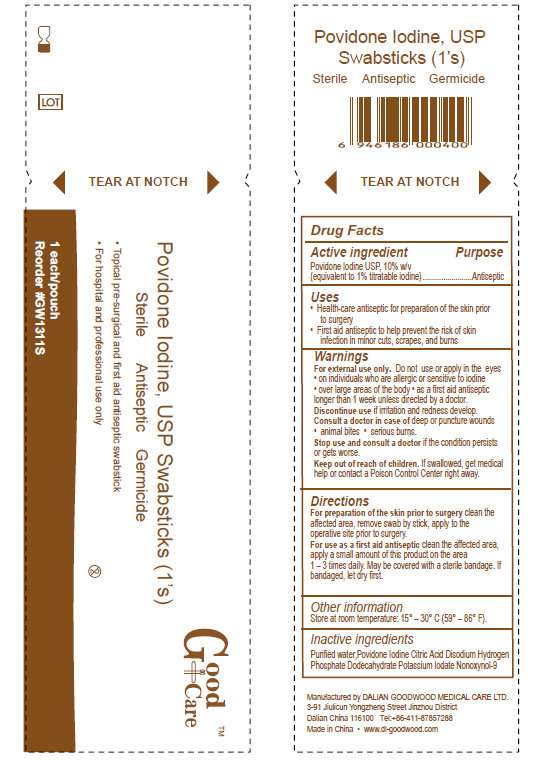
- Good Care Povidone Iodine, USP Swabsticks
- Active Ingredient
- Purpose
- Good Care Povidone Iodine Swabsticks Uses
- Warnings
- Directions
- Other Information
- Inactive Ingredients
- Manufactured by Dalian Goodwood Medical Care Ltd.
- Good Care Povidone Iodine, USP Swabsticks (1s) (50 pouches/box) (50685-009-01)
- Good Care Povidone Iodine, USP Swabsticks (2s) (25 pouches/box) (50685-009-02)
- Good Care Povidone Iodine, USP Swabsticks (3s) (25 pouches/box) (50685-009-03)
FULL PRESCRIBING INFORMATION
Good Care Povidone Iodine, USP Swabsticks
Active Ingredient
Povidone Iodine USP, 10%
Purpose
Antiseptic
Good Care Povidone Iodine Swabsticks Uses
- Health-care antiseptic for preparation of the skin prior to surgery
- First aid antiseptic to help prevent the risk of skin infection in minor cuts, scrapes, and burns
Warnings
- For external use only. Do not use or apply in the eyes
- on individuals who are allergic or sensitive to iodine
- over large areas of the body
- as a first aid antiseptic longer than 1 week unless directed by a doctor
Keep out of reach of children.
- If swallowed, get medical help or contact a Poison Control right away
Directions
Other Information
- Store at room temperature: 15 degrees - 30 degrees C (59 degrees - 86 degrees F)
Inactive Ingredients
Purified water, Povidone Iodine, Citric Acid Disodium Hydrogen Phosphate Dodecahydrate Potassium Iodate Nonoxynol
Manufactured by Dalian Goodwood Medical Care Ltd.
Made in China
Good Care Povidone Iodine, USP Swabsticks (1s) (50 pouches/box) (50685-009-01)

Good Care Povidone Iodine, USP Swabsticks (2s) (25 pouches/box) (50685-009-02)


Good Care Povidone Iodine, USP Swabsticks (3s) (25 pouches/box) (50685-009-03)


Good Care Povidone Iodine SwabsticksPOVIDONE-IODINE SWAB
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!