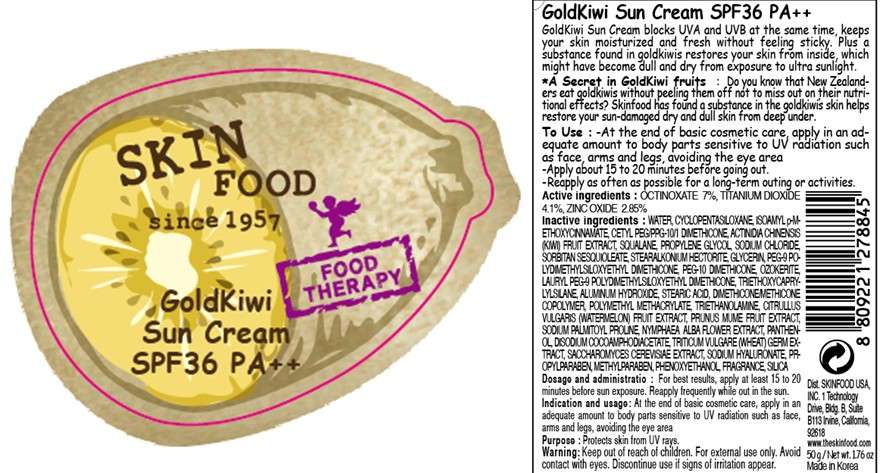
GOLDKIWI SUN
SKINFOOD CO., LTD.
SKINFOOD CO., LTD.
Drug Facts
FULL PRESCRIBING INFORMATION
Active ingredient
Active ingredients: OCTINOXATE 7%, TITANIUM DIOXIDE 4.1%, ZINC OXIDE 2.85%
Inactive ingredients:
WATER, CYCLOPENTASILOXANE, ISOAMYL p-METHOXYCINNAMATE, CETYL PEG/PPG-10/1 DIMETHICONE, ACTINIDIA CHINENSIS (KIWI) FRUIT EXTRACT, SQUALANE, PROPYLENE GLYCOL, SODIUM CHLORIDE, SORBITAN SESQUIOLEATE, STEARALKONIUM HECTORITE, GLYCERIN, PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE, PEG-10 DIMETHICONE, OZOKERITE, LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE, TRIETHOXYCAPRYLYLSILANE, ALUMINUM HYDROXIDE, STEARIC ACID, DIMETHICONE/METHICONE COPOLYMER, POLYMETHYL METHACRYLATE, TRIETHANOLAMINE, CITRULLUS VULGARIS (WATERMELON) FRUIT EXTRACT, PRUNUS MUME FRUIT EXTRACT, SODIUM PALMITOYL PROLINE, NYMPHAEA ALBA FLOWER EXTRACT, PANTHENOL, DISODIUM COCOAMPHODIACETATE, TRITICUM VULGARE (WHEAT) GERM EXTRACT, SACCHAROMYCES CEREVISIAE EXTRACT, SODIUM HYALURONATE, PROPYLPARABEN, METHYLPARABEN, PHENOXYETHANOL, FRAGRANCE, SILICA
Purpose
Purpose: Protects skin from UV rays.
Warnings:
For external use only.
Avoid contact with eyes.
Discontinue use if signs of irritation appear.
Keep out of reach of children:
Keep out of reach of children.
Uses
Indication and usage:
At the end of basic cosmetic care, apply in an adequate amount to body parts sensitive to UV radiation such as face, arms and legs, avoiding the eye area.
Dosage and administration:
For best results, apply at least 15 to 20 minutes before sun exposure.
Reapply frequently while out in the sun.
GOLDKIWI SUNOCTINOXATE CREAM
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||