glytone retexturize hand
Glytone retexturize hand
FULL PRESCRIBING INFORMATION
Active ingredient
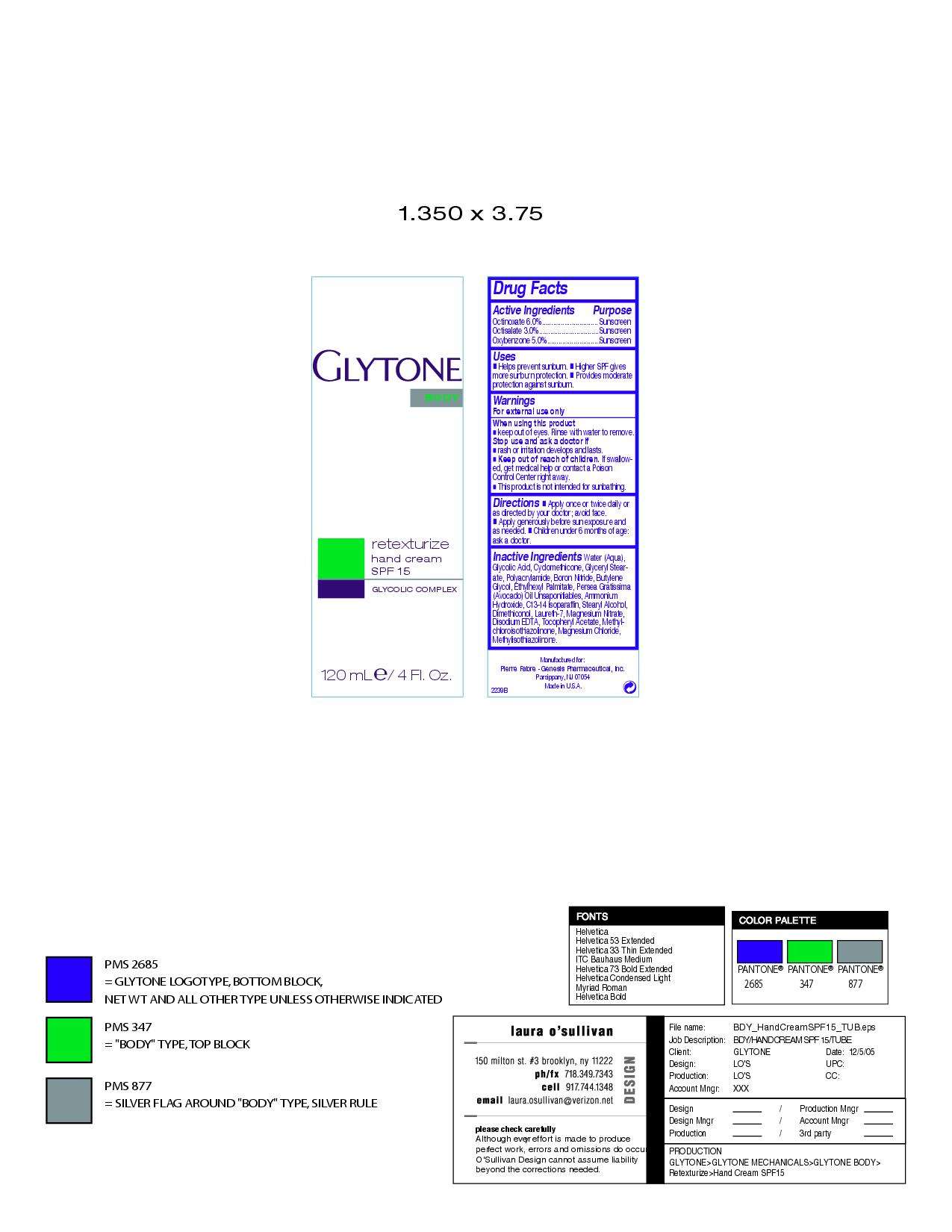
Active Ingredients Purpose
Octinoxate 6.0% Sunscreen
Octisalate 3.0% Sunscreen
Oxybenzone 5.0% Sunscreen
Inactive Ingredients
Water (Aqua), Glycolic Acid, Cyclomethicone, Glyceryl Stearate, Polyacrylamide, Boron Nitride, Butylene Glycol, Ethylhexly Palmitate, Persea Gratissima (Avocado) Oil Unsaponifiables, Ammoniium Hydroxide, C13-14 Isoparaffin, Stearly Alcohol, Dimethiconol, Laureth-7, Magnesium Nitrate, Disodium EDTA, Tocopheryl Acetate, Methyl-chloroisothiazolinone, Magnesium Chloride, Methylisothiazolinone.
Uses
Uses
Helps prevent sunburn
Higher SPF gives more sunburn protection
Porvides moderate protection against sunburn
Warnings
For external use only
When using this product
Keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if
rash or irritation develops and lasts.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
This product is not intended for sunbathing.
Directions
Apply once or twice a day or as directed by your doctor; avoid face.
Apply generously before sun exposure and as needed.
Children under 6 months of age: ask a doctor.
Glytone Body
retexturize hand cream SPF 15
glycolic complex
120 mL E/4 Fl. Oz.
glytone retexturize handoctinoxate, octisalate, oxybenzone CREAM
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||