Glytone acne treatment facial cleanser
Glytone acne treatment facial
FULL PRESCRIBING INFORMATION
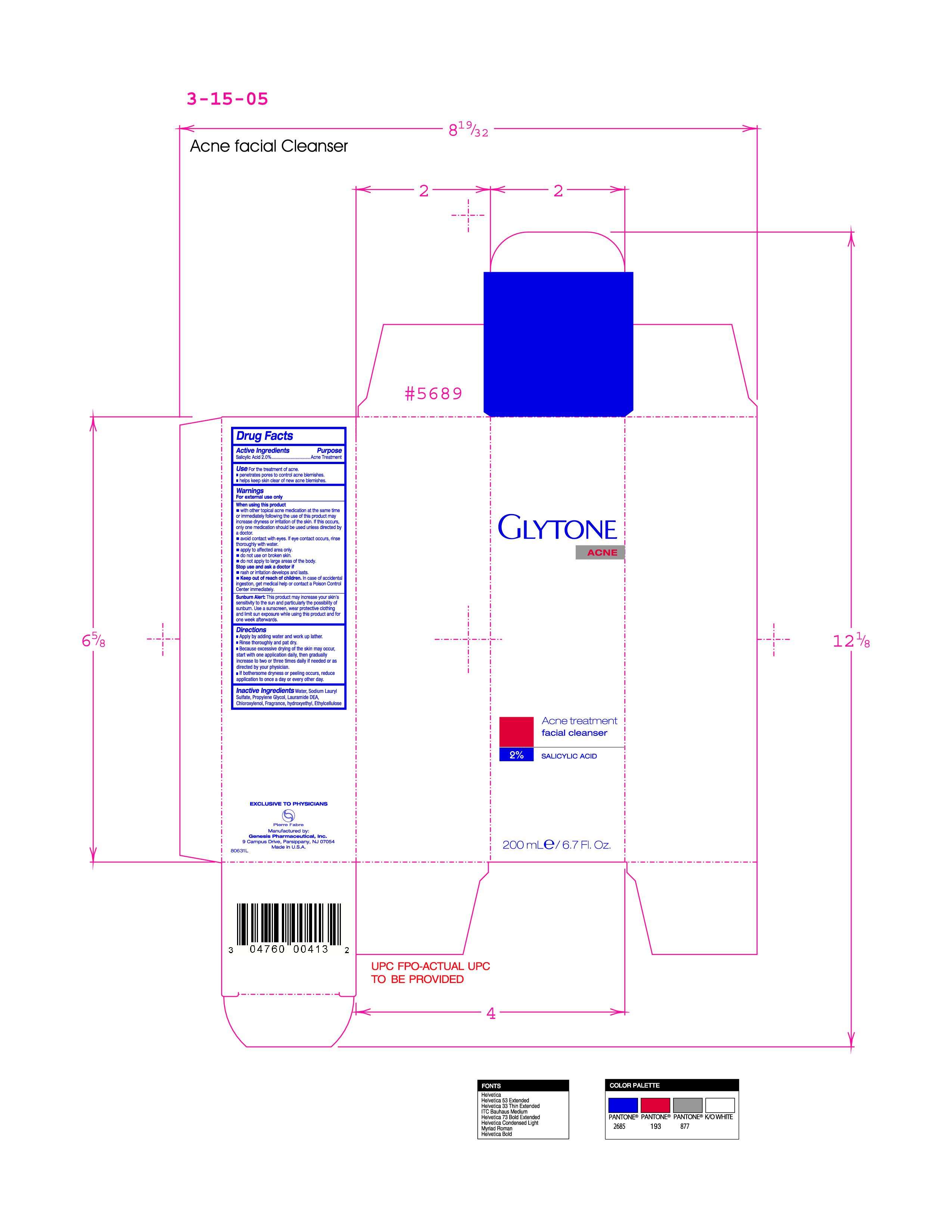
Inactive Ingredients water, sodium Lauryl Sulfate, Propylene Glycol, Lauramide DEA, Chloroxylenol, Fragrance, hydroxyethyl, Ethylcellulose
Directions
Apply by adding water and work up lather
Rinse thoroughly and pat dry
Because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by your physician
If bothersome dryness or peeling occurs, reduce application to once a day or every other day
Purpose
Purpose:
Acne Treatment
Keep out of reach of children. In case of accidental ingestion, get medical help or contact a Poison Control Center immediately.
Warnings
For external use only
When using this product
- with other topical acne medicatin at the same time or immediately following the use of this product may increase drynes or irritation of the skin.If this occurs, only one medication should be used unless directed by a doctor.
- avoid contact with eyes. If eye contact occurs, rinse thoroughly with water.
- apply to affected area only
- do not use on broken skin
- do not apply to large areas of the body
- rash or irritation develops and lasts
Active ingredient
Active Ingredient Salicylic Acid 2.0%
Uses
Directions
- Apply by adding water and work up lather.
- Rinse thoroughly and pat dry.
- Because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by your physician.
- If bothersome dryness or peeling occurs, reduce application to once a day or every other day.
Glytone Acne
Acne treatment
facial cleanser
2% salacylic acid
200mle/6.7floz
Glytone acne treatment facial cleansersalicylic acid LIQUID
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||