GILTUSS PEDIATRIC
Gil Pharmaceutical Corp
Hi-Tech Pharmacal Co., Inc.
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredients
- Purposes
- GILTUSS PEDIATRIC Uses
- Warnings
- Directions
- GILTUSS PEDIATRIC Other information
- Inactive ingredients
- Questions or comment?
- Package/Label Principal Display Panel
FULL PRESCRIBING INFORMATION
Active ingredients
(in each 1 mL)
Dextromethorphan HBr, 7.5 mg
Guaifenesin, 88 mg
Phenylephrine HCI, 2.5 mg
Purposes
Antitussive
Expectorant
Nasal Decongestant
GILTUSS PEDIATRIC Uses
- temporarily relieves cough due to minor throat and bronchial irritation as may occur with the common cold or inhaled irritants
- helps loosen phlegm (mucus) and thin bronchial secretions to make cough more productive
- temporarily relieves nasal congestion due to a cold
Warnings
Do not use
- in a child who is taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your child's prescription drug contains an MAOI, ask a doctor or pharmacist before giving this product.
Ask a doctor before use if you have
- a child who has heart disease, high blood pressure, thyroid disease, or diabetes unless directed by a doctor
- a cough with too much phlegm (mucus)
- a persistent or chronic cough such as occurs with asthma or if cough is accompanied by excessive phlegm (mucus) unless directed by a doctor
When using this product
- Do not exceed recommended dosage
Stop use and ask a doctor if
- nervousness, dizziness, or sleeplessness occur
- cough lasts for more than 7 days, comes back, or occurs with fever, rash or headache that lasts. These can be signs of a serious condition.
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children.
In case of accidental overdose, seek professional assistance or contact a Poison Control Center immediately.
Directions
- do not exceed recommended dosage
- take every 6 - 8 hours
- administer using the spill guard dispenser and syringe that's provided
| children 6 to under 12 years of age | 2 mL |
| children 2 to 6 years of age | 1 mL |
| children under 2 years of age | Consult a doctor |
GILTUSS PEDIATRIC Other information
- Tamper evident: Do not use if safety seal is broken or missing
- Store at controlled room temperature, between 15°- 30° C (59° - 86° F)
INSTRUCTIONS FOR USING THE ORAL LIQUID SPILL GUARD DISPENSER AND SYRINGE (INSTRUCCIONES PARA USAR EL "SPILL GUARD DISPENSER" Y LA JERINGUILLA PARA EL LIQUIDO ORAL)
1. Remove the child resistant cap and insert the Spill Guard Dispenser (B) into the bottle (A). Insert syringe (C) into Spill Guard Dispenser opening.
1. (Remueva la tapa resistente a niños. Introduzca en la botella (A) el "Spill Guard Dispenser" (B) Introduzca la jeringuilla (C) por la abertura del "Spill Guard Dispenser".)
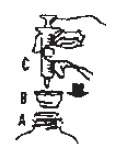
2. Turn bottle with Spill Guard Dispenser and syringe upside down and withdraw the required amount.
2. (Invierta la botella con el "Spill Guard Dispenser" y la jeringuilla y extragia la cantidad requerida.)
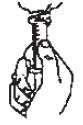
3. Return bottle to upright position. Remove the syringe and administer the medicine. Clean the syringe for next use. Leave Spill Guard Dispenser in bottle and replace child resistant cap.
3. (Vuelva a invertir toda la unidad a su posición original. Remueva la jeringuilla y administre la medicina. Lave la jeringuilla para su próximo uso. Deje puesto el "Spill Guard Dispenser" en la botella y ciérrela nuevamente con la tapa resistente a niños.)

Inactive ingredients
Artificial grape and vanilla flavor, bitter mask, citric acid, grapeskin extract, menthol, monoammonium glycyrrhizinate, polyethylene glycol, propylene glycol, purified water, sodium benzoate, sorbitol solution, sucralose. Sodium citrate may be used to adjust pH.
Questions or comment?
Call 1-877-746-9114, Mon. -Fri. 8:00 am thru 5:00 pm EST. Call your doctor for medical advice in the event of side effects.
Rev. 323:01 6/10
Package/Label Principal Display Panel
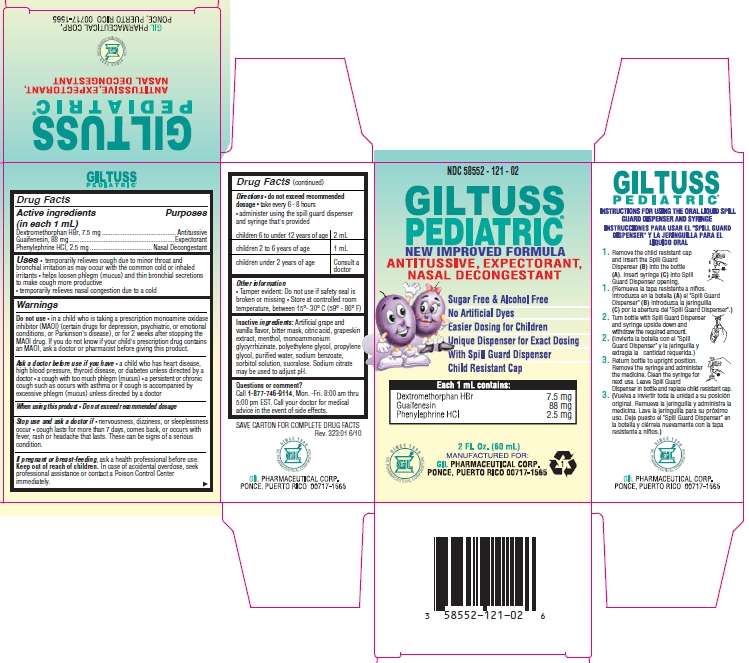
NDC 58552-121-02
GILTUSS PEDIATRIC ®
NEW IMPROVED FORMULA
ANTITUSSIVE, EXPECTORANT, NASAL DECONGESTANT
Sugar Free & Alcohol Free
No Artificial Dyes
Easier Dosing for Children
Unique Dispenser for Exact Dosing
With Spill Guard Dispenser
Child Resistant Cap
Each 1 mL contains:
Dextromethorphan HBr..................................7.5 mg
Guaifenesin......................................................88 mg
Phenylephrine HCl..........................................2.5 mg
2 Fl. Oz. (60 mL)
MANUFACTURED FOR:
GIL PHARMACEUTICAL CORP.
PONCE, PUERTO RICO 00717-1565
GILTUSS PEDIATRICdextromethorphan hydrobromide, guaifenesin, and phenylephrine hydrochloride SOLUTION/ DROPS
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||