Gabapentin
FULL PRESCRIBING INFORMATION: CONTENTS*
- GABAPENTIN DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACOKINETICS
- CLINICAL STUDIES
- INDICATIONS & USAGE
- GABAPENTIN CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- DRUG & OR LABORATORY TEST INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- GABAPENTIN ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- SPL MEDGUIDE
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
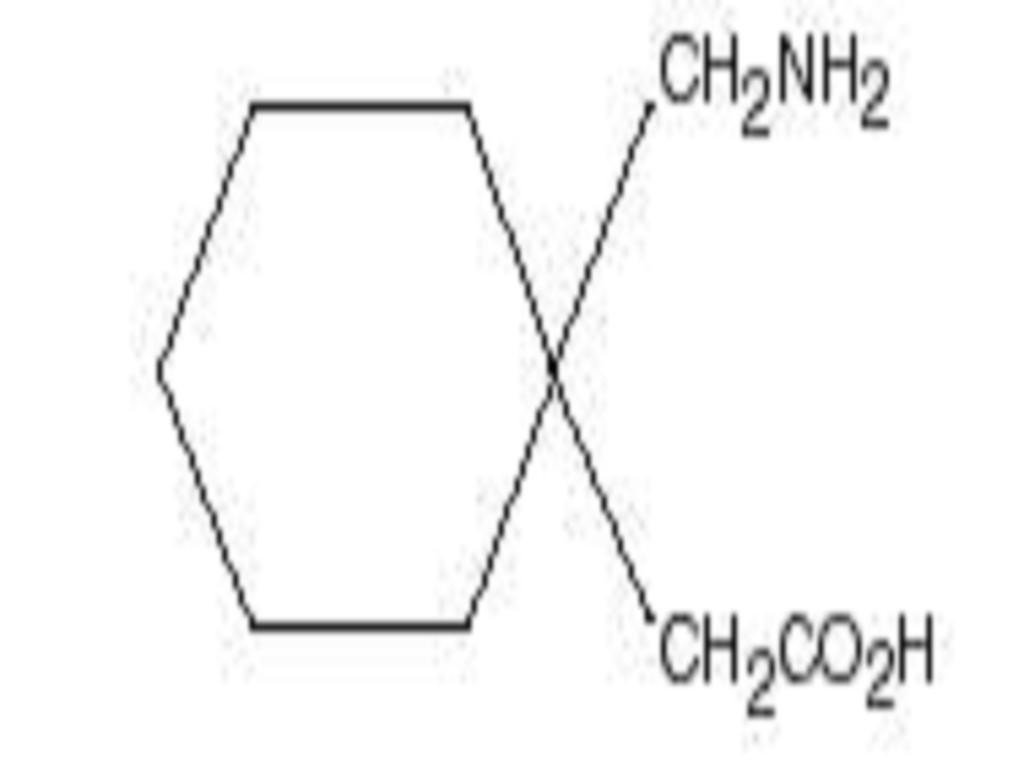
GABAPENTIN DESCRIPTION
CLINICAL PHARMACOLOGY
Mechanism of ActionPHARMACOKINETICS
Pharmacokinetics and Drug MetabolismOral Bioavailability
Distribution
Less than 3% of gabapentin circulates bound to plasma protein. The apparent volume of distribution of gabapentin after 150 mg intravenous administration is 586 L (MeanSD). In patients with epilepsy, steady-state predose (Cmin) concentrations of gabapentin in cerebrospinal fluid were approximately 20% of the corresponding plasma concentrations.
Elimination
Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans.
Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly proportional to creatinine clearance (see Special Populations, Adult Patients With Renal Insufficiency, below). In elderly patients, and in patients with impaired renal function, gabapentin plasma clearance is reduced. Gabapentin can be removed from plasma by hemodialysis.
Dosage adjustment in patients with compromised renal function or undergoing hemodialysis is recommended (see DOSAGE AND ADMINISTRATION, Table 6).
Special Populations
Adult Patients With Renal Insufficiency
Subjects (n = 60) with renal insufficiency (mean creatinine clearance ranging from 13 to 114 mL/min) were administered single 400 mg oral doses of gabapentin. The mean gabapentin half-life ranged from about 6.5 hours (patients with creatinine clearance > 60 mL/min) to 52 hours (creatinine clearance < 30 mL/min) and gabapentin renal clearance from about 90 mL/min (> 60 mL/min group) to about 10 mL/min (< 30 mL/min). Mean plasma clearance (CL/F) decreased from approximately 190 mL/min to 20 mL/min.
Dosage adjustment in adult patients with compromised renal function is necessary (see DOSAGE AND ADMINISTRATION). Pediatric patients with renal insufficiency have not been studied.
Hemodialysis
Dosage adjustment in patients undergoing hemodialysis is necessary (see DOSAGE AND ADMINISTRATION).
Hepatic Disease
Because gabapentin is not metabolized, no study was performed in patients with hepatic impairment.
Age
Pediatric
Gabapentin pharmacokinetics were determined in 48 pediatric subjects between the ages of 1 month and 12 years following a dose of approximately 10 mg/kg. Peak plasma concentrations were similar across the entire age group and occurred 2 to 3 hours postdose. In general, pediatric subjects between 1 month and < 5 years of age achieved approximately 30% lower exposure (AUC) than that observed in those 5 years of age and older. Accordingly, oral clearance normalized per body weight was higher in the younger children. Apparent oral clearance of gabapentin was directly proportional to creatinine clearance. Gabapentin elimination half-life averaged 4.7 hours and was similar across the age groups studied.
A population pharmacokinetic analysis was performed in 253 pediatric subjects between 1 month and 13 years of age. Patients received 10 to 65 mg/kg/day given TID. Apparent oral clearance (CL/F) was directly proportional to creatinine clearance and this relationship was similar following a single dose and at steady state. Higher oral clearance values were observed in children < 5 years of age compared to those observed in children 5 years of age and older, when normalized per body weight. The clearance was highly variable in infants < 1 year of age. The normalized CL/F values observed in pediatric patients 5 years of age and older were consistent with values observed in adults after a single dose. The oral volume of distribution normalized per body weight was constant across the age range.
These pharmacokinetic data indicate that the effective daily dose in pediatric patients with epilepsy ages 3 and 4 years should be 40 mg/kg/day to achieve average plasma concentrations similar to those achieved in patients 5 years of age and older receiving gabapentin at 30 mg/kg/day (see DOSAGE AND ADMINISTRATION).
Gender
Although no formal study has been conducted to compare the pharmacokinetics of gabapentin in men and women, it appears that the pharmacokinetic parameters for males and females are similar and there are no significant gender differences.
Race
Pharmacokinetic differences due to race have not been studied. Because gabapentin is primarily renally excreted and there are no important racial differences in creatinine clearance, pharmacokinetic differences due to race are not expected.
CLINICAL STUDIES
Postherpetic Neuralgia
Table 1. Controlled PHN Studies: Duration, Dosages, and Number of Patients
*Given in 3 divided doses (TID)StudyStudy DurationGabapentin (mg/day)*Target DosePatients Receiving GabapentinPatients Receiving Placebo18 weeks360011311627 weeks1800, 2400223111Total336227Each study included a 1 week baseline during which patients were screened for eligibility and a 7 or 8 week double-blind phase (3 or 4 weeks of titration and 4 weeks of fixed dose). Patients initiated treatment with titration to a maximum of 900 mg/day gabapentin over 3 days. Dosages were then to be titrated in 600 to 1200 mg/day increments at 3 to 7 day intervals to target dose over 3 to 4 weeks. In Study 1, patients were continued on lower doses if not able to achieve the target dose. During baseline and treatment, patients recorded their pain in a daily diary using an 11 point numeric pain rating scale ranging from 0 (no pain) to 10 (worst possible pain). A mean pain score during baseline of at least 4 was required for randomization (baseline mean pain score for Studies 1 and 2 combined was 6.4). Analyses were conducted using the ITT population (all randomized patients who received at least one dose of study medication).
Both studies showed significant differences from placebo at all doses tested.
A significant reduction in weekly mean pain scores was seen by Week 1 in both studies, and significant differences were maintained to the end of treatment. Comparable treatment effects were observed in all active treatment arms. Pharmacokinetic/pharmacodynamic modeling provided confirmatory evidence of efficacy across all doses. Figures 1 and 2 show these changes for Studies 1 and 2.
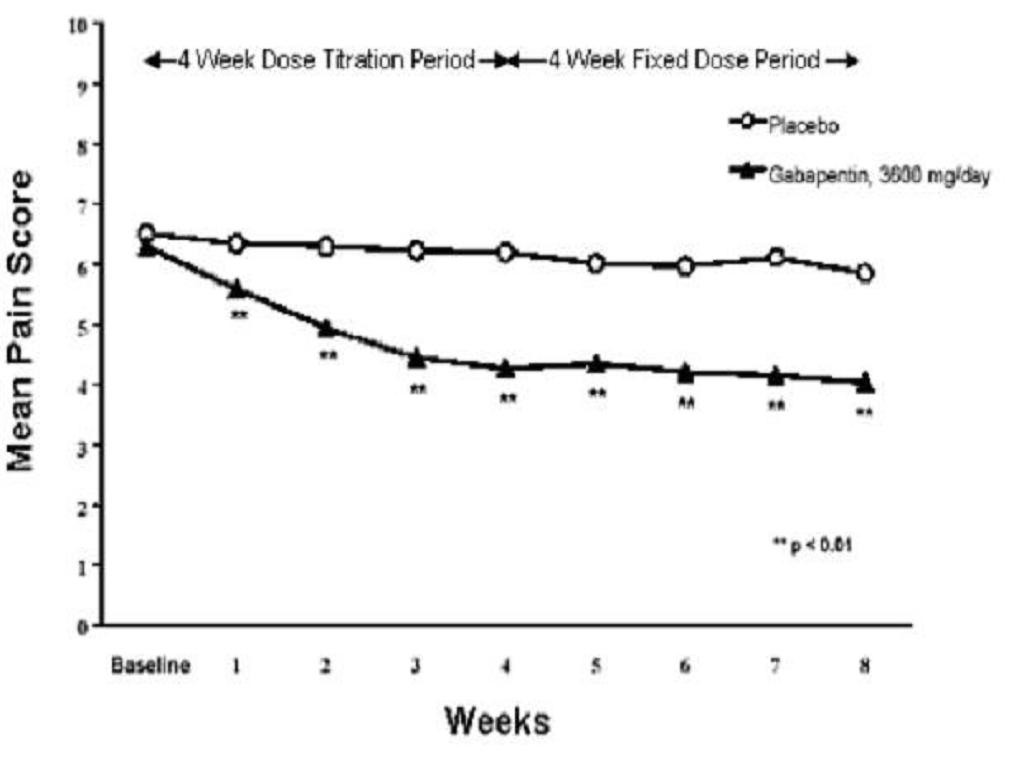
Figure 1. Weekly Mean Pain Scores (Observed Cases in ITT Population): Study 1
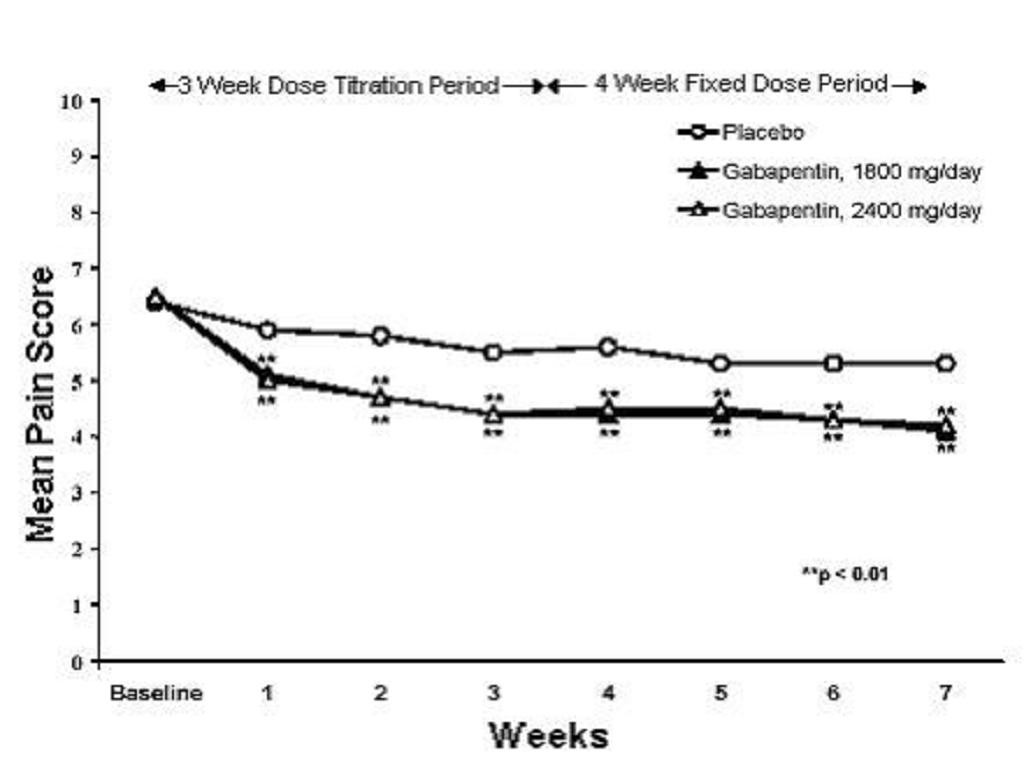
Figure 2. Weekly Mean Pain Scores (Observed Cases in ITT Population): Study 2
The proportion of responders (those patients reporting at least 50% improvement in endpoint pain score compared with baseline) was calculated for each study ( Figure 3).
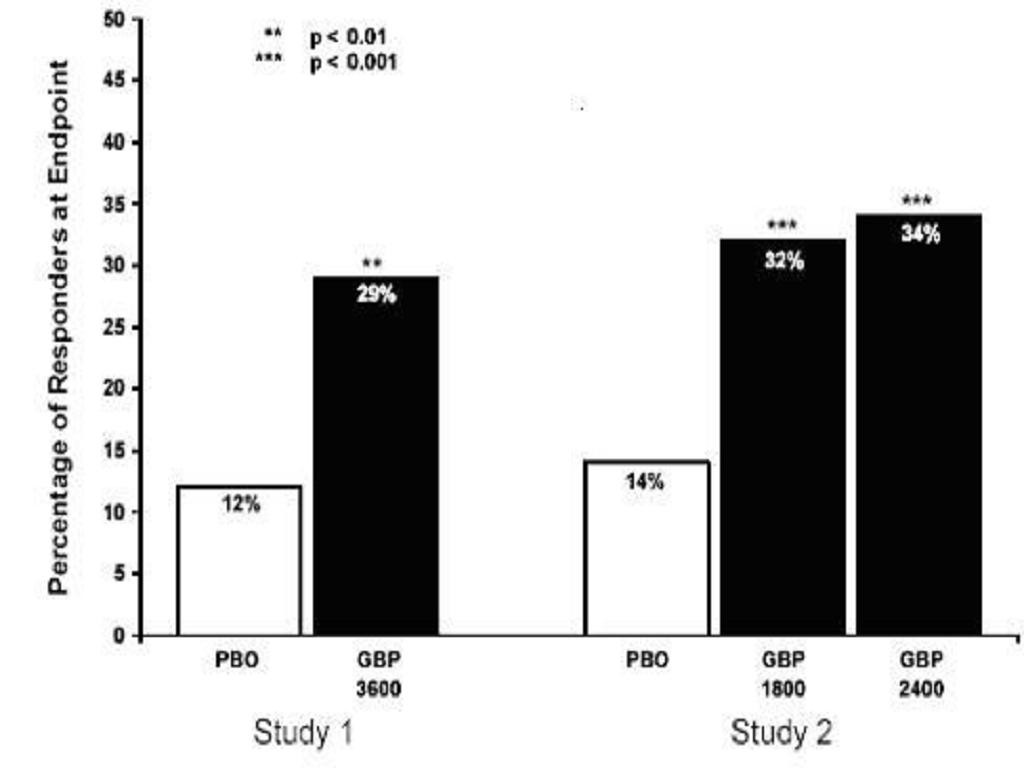
Figure 3. Proportion of Responders (patients with50% reduction in pain score) at Endpoint: Controlled PHN Studies
Epilepsy
The effectiveness of gabapentin as adjunctive therapy (added to other antiepileptic drugs) was established in multicenter placebo-controlled, double-blind, parallel-group clinical trials in adult and pediatric patients (3 years and older) with refractory partial seizures.
Evidence of effectiveness was obtained in three trials conducted in 705 patients (age 12 years and above) and one trial conducted in 247 pediatric patients (3 to 12 years of age). The patients enrolled had a history of at least 4 partial seizures per month in spite of receiving one or more antiepileptic drugs at therapeutic levels and were observed on their established antiepileptic drug regimen during a 12 week baseline period (6 weeks in the study of pediatric patients). In patients continuing to have at least 2 (or 4 in some studies) seizures per month, gabapentin or placebo was then added on to the existing therapy during a 12 week treatment period. Effectiveness was assessed primarily on the basis of the percent of patients with a 50% or greater reduction in seizure frequency from baseline to treatment (the "responder rate") and a derived measure called response ratio, a measure of change defined as (T - B)/(T + B), where B is the patient's baseline seizure frequency and T is the patient
A third study compared gabapentin 900 mg/day divided TID (n = 111) and placebo (n = 109). An additional gabapentin 1200 mg/day dosage group (n = 52) provided dose-response data. A statistically significant difference in responder rate was seen in the gabapentin 900 mg/day group (22%) compared to that in the placebo group (10%). Response ratio was also statistically significantly superior in the gabapentin 900 mg/day group (-0.119) compared to that in the placebo group (-0.027), as was response ratio in 1200 mg/day gabapentin (-0.184) compared to placebo.
Analyses were also performed in each study to examine the effect of gabapentin on preventing secondarily generalized tonic-clonic seizures. Patients who experienced a secondarily generalized tonic-clonic seizure in either the baseline or in the treatment period in all three placebo-controlled studies were included in these analyses. There were several response ratio comparisons that showed a statistically significant advantage for gabapentin compared to placebo and favorable trends for almost all comparisons.
In two of the three controlled studies, more than one dose of gabapentin was used. Within each study the results did not show a consistently increased response to dose. However, looking across studies, a trend toward increasing efficacy with increasing dose is evident (see Figure 4).

Figure 4. Responder Rate in Patients Receiving Gabapentin Expressed as a Difference From Placebo by Dose and Study: Adjunctive Therapy Studies in Patients12 Years of Age With Partial Seizures
In the figure, treatment effect magnitude, measured on the Y axis in terms of the difference in the proportion of gabapentin and placebo assigned patients attaining a 50% or greater reduction in seizure frequency from baseline, is plotted against the daily dose of gabapentin administered (X axis).
Although no formal analysis by gender has been performed, estimates of response (Response Ratio) derived from clinical trials (398 men, 307 women) indicate no important gender differences exist. There was no consistent pattern indicating that age had any effect on the response to gabapentin. There were insufficient numbers of patients of races other than Caucasian to permit a comparison of efficacy among racial groups.
A fourth study in pediatric patients age 3 to 12 years compared 25 to 35 mg/kg/day gabapentin (n = 118) with placebo (n = 127). For all partial seizures in the intent-to-treat population, the response ratio was statistically significantly better for the gabapentin group (-0.146) than for the placebo group (-0.079). For the same population, the responder rate for gabapentin (21%) was not significantly different from placebo (18%).
A study in pediatric patients age 1 month to 3 years compared 40 mg/kg/day gabapentin (n = 38) with placebo (n = 38) in patients who were receiving at least one marketed antiepileptic drug and had at least one partial seizure during the screening period (within 2 weeks prior to baseline). Patients had up to 48 hours of baseline and up to 72 hours of double-blind video EEG monitoring to record and count the occurrence of seizures. There were no statistically significant differences between treatments in either the response ratio or responder rate.
INDICATIONS & USAGE
Postherpetic NeuralgiaEpilepsy
GABAPENTIN CONTRAINDICATIONS
WARNINGS
Suicidal Behavior and IdeationNeuropsychiatric Adverse EventsPediatric Patients 3 to 12 Years of Age
Withdrawal Precipitated Seizure, Status Epilepticus
Tumorigenic Potential
Sudden and Unexplained Death in Patients with Epilepsy
PRECAUTIONS
INFORMATION FOR PATIENTS
LABORATORY TESTS
DRUG INTERACTIONS
Phenytoin
Carbamazepine
Valproic Acid
Phenobarbital
Naproxen
Coadministration (n = 18) of naproxen sodium capsules (250 mg) with gabapentin (125 mg) appears to increase the amount of gabapentin absorbed by 12% to 15%. Gabapentin had no effect on naproxen pharmacokinetic parameters. These doses are lower than the therapeutic doses for both drugs. The magnitude of interaction within the recommended dose ranges of either drug is not known.
Hydrocodone
Morphine
A literature article reported that when a 60 mg controlled-release morphine capsule was administered 2 hours prior to a 600 mg gabapentin capsule (n = 12), mean gabapentin AUC increased by 44% compared to gabapentin administered without morphine (see PRECAUTIONS). Morphine pharmacokinetic parameter values were not affected by administration of gabapentin 2 hours after morphine. The magnitude of interaction at other doses is not known.
Cimetidine
In the presence of cimetidine at 300 mg QID (n = 12) the mean apparent oral clearance of gabapentin fell by 14% and creatinine clearance fell by 10%. Thus cimetidine appeared to alter the renal excretion of both gabapentin and creatinine, an endogenous marker of renal function. This small decrease in excretion of gabapentin by cimetidine is not expected to be of clinical importance. The effect of gabapentin on cimetidine was not evaluated.
Oral Contraceptive
Antacid (Aluminum Hydroxide and Magnesium Hydroxide)
Aluminum hydroxide and magnesium hydroxide reduced the bioavailability of gabapentin (n = 16) by about 20%. This decrease in bioavailability was about 5% when gabapentin was administered 2 hours after aluminum hydroxide and magnesium hydroxide. It is recommended that gabapentin be taken at least 2 hours following aluminum hydroxide and magnesium hydroxide administration.
Effect of Probenecid
Probenecid is a blocker of renal tubular secretion. Gabapentin pharmacokinetic parameters without and with probenecid were comparable. This indicates that gabapentin does not undergo renal tubular secretion by the pathway that is blocked by probenecid.
DRUG & OR LABORATORY TEST INTERACTIONS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Teratogenic EffectsPregnancy Category C
NURSING MOTHERS
PEDIATRIC USE
GERIATRIC USE
GABAPENTIN ADVERSE REACTIONS
Postherpetic NeuralgiaIncidence in Controlled Clinical Trials
**
Epilepsy
Incidence in Controlled Clinical Trials
*Amblyopia was often described as blurred visionBody System/Adverse EventGabapentin**4.21.1Laboratory DeviationsWBC decreased1.10.5Other events in more than 1% of patients > 12 years of age but equally or more frequent in the placebo group included: headache, viral infection, fever, nausea and/or vomiting, abdominal pain, diarrhea, convulsions, confusion, insomnia, emotional lability, rash, acne.
Among the treatment-emergent adverse events occurring at an incidence of at least 10% of gabapentin-treated patients, somnolence and ataxia appeared to exhibit a positive dose-response relationship.
The overall incidence of adverse events and the types of adverse events seen were similar among men and women treated with gabapentin. The incidence of adverse events increased slightly with increasing age in patients treated with either gabapentin or placebo. Because only 3% of patients (28/921) in placebo-controlled studies were identified as nonwhite (black or other), there are insufficient data to support a statement regarding the distribution of adverse events by race.
Table 5 lists treatment-emergent signs and symptoms that occurred in at least 2% of gabapentin-treated patients age 3 to 12 years of age with epilepsy participating in placebo-controlled trials and were numerically more common in the gabapentin group. Adverse events were usually mild to moderate in intensity.
Table 5. Treatment-Emergent Adverse Event Incidence in Pediatric Patients Age 3 to 12 Years in a Controlled Add-On Trial (Events in at Least 2% of Gabapentin Patients and Numerically More Frequent Than in the Placebo Group)
***
Other Adverse Events Observed During All Clinical Trials
Clinical Trials in Adults and Adolescents (Except Clinical Trials in Neuropathic Pain)
Body as a Whole
Cardiovascular System
Digestive System
Endocrine System
Hematologic and Lymphatic System
Musculoskeletal System
Nervous System
Respiratory System
Dermatological
Urogenital System
Special Senses
Clinical Trials in Pediatric Patients With Epilepsy
Clinical Trials in Adults with Neuropathic Pain of Various Etiologies
Body as a Whole
Cardiovascular System
Digestive System
Endocrine System
Hemic and Lymphatic System
Metabolic and Nutritional
Musculoskeletal
Nervous System
Respiratory System
Skin and Appendages
Special Senses
Urogenital System
Postmarketing and Other Experience
DRUG ABUSE AND DEPENDENCE
OVERDOSAGE
DOSAGE & ADMINISTRATION
Postherpetic Neuralgia
Epilepsy
Patients > 12 Years of Age
Pediatric Patients Age 3 to 12 Years
Dosage in Renal Impairment
*Patients on hemodialysis should receive maintenance doses based on estimates of creatinine clearance as indicated in the upper portion of the table and a supplemental post-hemodialysis dose administered after each 4 hours of hemodialysis as indicated in the lower portion of the table.Renal Function Creatinine ClearanceTotal Daily Dose RangeDose Regimen(mL/min)(mg/day)(mg)60900 to 3600300 TID400 TID600 TID800 TID1200 TID> 30 to 59400 to 1400200 BID300 BID400 BID500 BID700 BID> 15 to 29200 to 700200 QD300 QD400 QD500 QD700 QD15*Hemodialysis125150200250350The use of gabapentin tablets USP in patients < 12 years of age with compromised renal function has not been studied.
Dosage in Elderly
Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and dose should be adjusted based on creatinine clearance values in these patients.
HOW SUPPLIED
SPL MEDGUIDE
-
● thoughts about suicide or dying
-
● attempts to commit suicide
-
● new or worse depression
-
● new or worse anxiety
-
● feeling agitated or restless
-
● panic attacks
-
● trouble sleeping (insomnia)
-
● new or worse irritability
-
● acting aggressive, being angry, or violent
-
● acting on dangerous impulses
-
● an extreme increase in activity and talking (mania)
-
● other unusual changes in behavior or mood
-
● How can I watch for early symptoms of suicidal thoughts and actions?
-
● Keep all follow-up visits with your healthcare provider as scheduled.
-
● Call your healthcare provider between visits as needed, especially if you are worried about symptoms.
-
● Suicidal thoughts or actions can be caused by things other than medicines. If you have suicidal thoughts or actions, your healthcare provider may check for other causes.
-
● Partial seizures when taken together with other medicines in adults and children 3 years of age and older.
-
● Who should not take gabapentin?
-
● have or have had depression, mood problems, or suicidal thoughts or behavior
-
● are pregnant or plan to become pregnant. It is not known if gabapentin can harm your unborn baby. Tell your healthcare provider right away if you become pregnant while taking gabapentin. You and your healthcare provider will decide if you should take gabapentin while you are pregnant.
-
● o If you become pregnant while taking gabapentin, talk to your healthcare provider about registering with the North American Antiepileptic Drug (NAAED) Pregnancy Registry. The purpose of this registry is to collect information about the safety of antiepileptic drugs during pregnancy. You can enroll in this registry by calling 1-888-233-2334.
-
● Tell your healthcare provider about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements.
-
● -Do not change your dose of gabapentin without talking to your healthcare provider. If you break a tablet in half the unused half of the tablet should be taken at your next scheduled dose. Half tablets not used within several days of breaking should be thrown away.
-
● If you take too much gabapentin, call your healthcare provider or your local Poison Control Center right away.
-
● What should I avoid while taking gabapentin?
-
● Do not drive, operate heavy machinery, or do other dangerous activities until you know how gabapentin affects you. Gabapentin can slow your thinking and motor skills.
-
● What are the possible side effects of gabapentin
-
● The most common side effects of gabapentin include:
-
● dizziness
-
● lack of coordination
-
● viral infection
-
● feeling drowsy
-
● feeling tired
-
● fever
-
● jerky movements
-
● difficulty with speaking
-
● temporary loss of memory (amnesia)
-
● tremor
-
● difficulty with coordination
-
● double vision
-
● unusual eye movement
-
● Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
-
● Keep gabapentin and all medicines out of the reach of children.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION


GabapentinGabapentin TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!