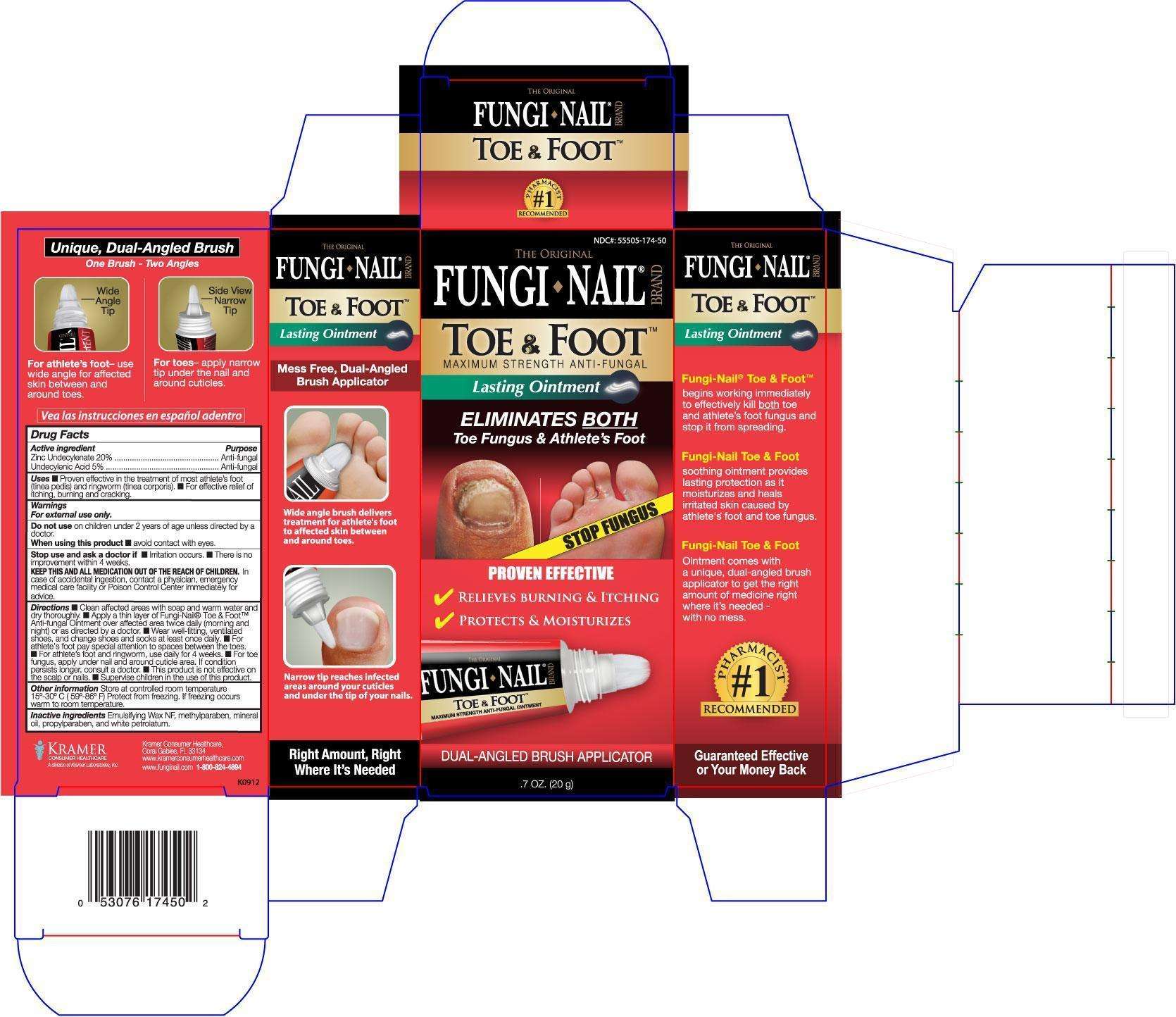
Fungi Nail Toe and Foot
FULL PRESCRIBING INFORMATION
Active ingredient
Undecylenic acid 5%
Zinc Undecylenate 20%
Purpose
Antifungal
KEEP THIS AND ALL MEDICATION OUT OF THE REACH OF CHILDREN. In
case of accidental ingestion, contact a physician, emergency
medical care facility or Poison Control Center immediately for
advice.
Uses
Uses ■ Proven effective in the treatment of most athlete’s foot
(tinea pedis) and ringworm (tinea corporis). ■ For effective relief of
itching, burning and cracking.
Directions ■ Clean affected areas with soap and warm water and
dry thoroughly. ■ Apply a thin layer of Fungi-Nail® Toe & Foot™
Anti-fungal Ointment over affected area twice daily (morning and
night) or as directed by a doctor. ■ Wear well-fitting, ventilated
shoes, and change shoes and socks at least once daily. ■ For
athlete’s foot pay special attention to spaces between the toes.
■ For athlete’s foot and ringworm, use daily for 4 weeks. ■ For toe
fungus, apply under nail and around cuticle area. If condition
persists longer, consult a doctor. ■ This product is not effective on
the scalp or nails. ■ Supervise children in the use of this product.
Emulsifying wax NF, methylparaben, mineral oil, propylparaben, white petrolatum
Warnings
For external use only.
Do not use on children under 2 years of age unless directed by a
doctor.
When using this product ■ avoid contact with eyes.
Fungi Nail Toe and FootZinc Undecylenate Undecylenic acid OINTMENT
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||