Forfivo
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use FORFIVO XL safely and effectively. See full prescribing information for FORFIVO XL. FORFIVO XL (bupropion hydrochloride extended-release tablets), for oral useInitial U.S. Approval: 1985BOXED WARNINGWARNING: SUICIDALITY and ANTIDEPRESSANT DRUGS; PSYCHIATRIC EVENTS and SMOKING CESSATION See full prescribing information for complete boxed warning Increased risk of suicidal thinking and behavior in children, adolescents, and young adults taking antidepressants for Major Depressive Disorder (MDD) and other psychiatric disorders. Closely monitor for clinical worsening and suicidal thinking and behavior (5.1). Serious neuropsychiatric events, including depression, suicidal ideation, suicide attempt, and completed suicide have been reported in patients taking bupropion for smoking cessation. Stop bupropion and contact a healthcare provider immediately if agitation, hostility, depressed mood, or changes in thinking or behavior that are not typical for the patient are observed, or if the patient develops suicidal ideation or suicidal behavior (5.2). INDICATIONS AND USAGEFORFIVO XL is an aminoketone indicated for the treatment of major depressive disorder (1). The efficacy was established in two 4 week trials, one 6 week trial with bupropion immediate release formulation and one maintenance trial with bupropion sustained-release formulation, all in adults (14).DOSAGE AND ADMINISTRATION Use one tablet (450 mg) once daily without regard to food (2.1). Swallow the tablet whole. Do not chew, divide, or crush (2.1). Do not initiate treatment with FORFIVO XL. Use another bupropion formulation for initial dose titration (2.2). Can be used in patients who are receiving 300 mg/day of another bupropion formulation for at least 2 weeks, and require a dosage of 450 mg/day (2.2), Patients who are currently being treated with other bupropion products at 450 mg/day can be switched to equivalent dose of FORFIVO XL once daily (2.2). DOSAGE FORMS AND STRENGTHS Extended-release tablets: 450 mg (3) CONTRAINDICATIONS Seizure disorder (4) Current use of other bupropion products (4) Current or prior diagnosis of bulimia or anorexia nervosa (4) Abrupt discontinuation of alcohol or sedatives (4) Use with monoamine oxidase (MAO) inhibitor: stop at least 2 weeks prior to bupropion use (4) Known hypersensitivity to bupropion or other ingredients of FORFIVO XL. (4, 5.9) WARNINGS AND PRECAUTIONS Activation of mania/ hypomania: Screen patients for bipolar disorder and monitor for manic symptoms. (5.3) Seizure risk: The risk is dose-dependent. Discontinue if seizure occurs (4, 5.4, 7.6) Psychosis and other neuropsychiatric events: Discontinue if these events occur. (5.5) Severe hypertension: Monitor blood pressure periodically. (5.6) Side EffectsMost common adverse reactions are (incidence ≥ 5%; ≥ 2 times placebo rate): Dry mouth, nausea, insomnia, dizziness, pharyngitis, abdominal pain, agitation, anxiety, tremor, palpitation, sweating, tinnitus, myalgia, anorexia, urinary frequency, rash (6.1) To report SUSPECTED ADVERSE REACTIONS, contact Edgemont Pharmaceuticals, LLC at 1-888- 594-4332 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONS Ticlopidine or clopidogrel: May increase bupropion exposure. Coadministration of FORFIVO XL with ticlopidine or clopidogrel is not recommended (7.1) Drugs metabolized by CYP2D6 (e.g., desipramine, paroxetine, fluoxetine, sertraline, venlafaxine): Consider dose reduction when using with bupropion. Bupropion & hydroxybupropion inhibit CYP2D6 (7.2) Nicotine transdermal system: Monitor for severe hypertension (5.6, 7.3) Drug laboratory test interactions: May cause false-positive urine immunoassay screening test results for amphetamines (7.4). USE IN SPECIFIC POPULATIONS Pregnancy: Use only if the potential benefit justifies the potential risk to the fetus (8.1) Nursing: Can be secreted in human milk. A decision whether to discontinue nursing or to discontinue the drug, should be made taking into account the importance of the drug to the mother. (8.3). Renal Impairment: Since there is no lower dose strength for FORFIVO XL, FORFIVO XL is not recommended in patients with renal impairment (8.6) Hepatic Impairment: Since there is no lower dose strength for FORFIVO XL, FORFIVO XL is not recommended in patients with hepatic impairment (8.7)
FULL PRESCRIBING INFORMATION: CONTENTS*
- WARNING: SUICIDALITY and ANTIDEPRESSANT DRUGS; PSYCHIATRIC EVENTS and SMOKING CESSATION
- 1 FORFIVO INDICATIONS AND USAGE
- 2 FORFIVO DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 FORFIVO CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 5.1 Clinical Worsening and Suicide Risk in Treating Psychiatric Disorder
- 5.2 Neuropsychiatric Symptoms and Suicide Risk in Smoking Cessation Treatment
- 5.3 Activation of Mania/Hypomania
- 5.4 Seizures
- 5.5 Psychosis and Other Neuropsychiatric Events
- 5.6 Severe Hypertension
- 5.7 Agitation and Insomnia
- 5.8 Altered Appetite and Weight
- 5.9 Hypersensitivity Reactions
- 6 FORFIVO ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 9 DRUG ABUSE AND DEPENDENCE
- 10 OVERDOSAGE
- 11 FORFIVO DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
- MEDICATION GUIDE
- PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
WARNING: SUICIDALITY and ANTIDEPRESSANT DRUGS; PSYCHIATRIC EVENTS and SMOKING CESSATION
SUICIDALITY and ANTIDEPRESSANT DRUGS:
Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of FORFIVO XL or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. FORFIVO XL is not approved for use in pediatric patients [see Warnings and Precautions (5.1)].
PSYCHIATRIC EVENTS and SMOKING CESSATION:
FORVIVO XL is not approved for smoking cessation treatment, but bupropion under the name ZYBAN® is approved for this use. Serious neuropsychiatric events, including but not limited to depression, suicidal ideation, suicide attempt, and completed suicide have been reported in patients taking bupropion for smoking cessation. Some cases may have been complicated by the symptoms of nicotine withdrawal in patients who stopped smoking. Depressed mood may be a symptom of nicotine withdrawal. Depression, rarely including suicidal ideation, has been reported in smokers undergoing a smoking cessation attempt without medication. However, some of these symptoms have occurred in patients taking bupropion who continued to smoke.
All patients being treated with bupropion for smoking cessation treatment should be observed for neuropsychiatric symptoms including changes in behavior, hostility, agitation, depressed mood, and suicide-related events, including ideation, behavior, and attempted suicide. These symptoms, as well as worsening of pre-existing psychiatric illness and completed suicide have been reported in some patients attempting to quit smoking while taking ZYBAN in the postmarketing experience. When symptoms were reported, most were during treatment with ZYBAN, but some were following discontinuation of treatment with ZYBAN. These events have occurred in patients with and without pre-existing psychiatric disease; some have experienced worsening of their psychiatric illnesses. Patients with serious psychiatric illness such as schizophrenia, bipolar disorder, and major depressive disorder did not participate in the premarketing studies of ZYBAN.
Advise patients and caregivers that the patient using bupropion for smoking cessation should stop taking bupropion and contact a healthcare provider immediately if agitation, hostility, depressed mood, or changes in thinking or behavior that are not typical for the patient are observed, or if the patient develops suicidal ideation or suicidal behavior. In many postmarketing cases, resolution of symptoms after discontinuation of ZYBAN was reported, although in some cases the symptoms persisted; therefore, ongoing monitoring and supportive care should be provided until symptoms resolve.
The risks of using bupropion for smoking cessation should be weighed against the benefits of its use. ZYBAN has been demonstrated to increase the likelihood of abstinence from smoking for as long as 6 months compared to treatment with placebo. The health benefits of quitting smoking are immediate and substantial [see Warnings and Precautions (5.2) and Patient Counseling Information (17)].
1 INDICATIONS AND USAGE
FORFIVO XL (bupropion hydrochloride extended-release tablets) is indicated for the treatment of major depressive disorder (MDD).
The efficacy in the treatment of MDD was established in two 4-week and one 6-week and one maintenance trial in adult patients whose diagnoses corresponded most closely to the Major Depression category of the APA Diagnostic and Statistical Manual (DSM) [see Clinical Studies (14) ].
A major depressive episode (DSM-IV) implies the presence of 1) depressed mood or 2) loss of interest or pleasure; in addition, at least 5 of the following symptoms have been present during the same 2-week period and represent a change from previous functioning: depressed mood, markedly diminished interest or pleasure in usual activities, significant change in weight and/or appetite, insomnia or hypersomnia, psychomotor agitation or retardation, increased fatigue, feelings of guilt or worthlessness, slowed thinking or impaired concentration, a suicide attempt, or suicidal ideation.
The efficacy of bupropion in pediatric population has not been established.
The physician who elects to use FORFIVO XL for extended periods should periodically reevaluate the long-term usefulness of the drug for the individual patient.
2 DOSAGE AND ADMINISTRATION
2.1 Instructions for Use
One tablet (450 mg) of FORFIVO XL should be taken once daily without regard to meals. FORFIVO XL tablet should be swallowed whole and not crushed, divided, or chewed.
2.2 Initial Treatment with FORFIVO XL
Do not initiate treatment with FORFIVO XL because 450 mg tablet is the only available dose formulation. Use another bupropion formulation for initial dose titration (referring to prescribing information of other bupropion products).
FORFIVO XL can be used in patients who are receiving 300 mg/day of another bupropion formulation for at least 2 weeks, and require a dosage of 450 mg/day.
Patients who are currently being treated with other bupropion products at 450 mg/day can be switched to equivalent dose of FORFIVO XL once daily.
2.3 Maintenance Treatment with FORFIVO XL
It is generally agreed that acute episodes of depression require several months or longer of sustained pharmacological therapy beyond response to the acute episode. It is unknown whether or not the 450 mg dose needed to achieve an initial response is identical to the dose needed for maintenance treatment. Patients should be periodically reassessed to determine the need for maintenance treatment and the appropriate dose for such treatment.
2.4 Patients with Impaired Hepatic Function
Since there is no lower dose strength for FORFIVO XL, FORFIVO XL is not recommended in patients with hepatic impairment [see Use in Specific Population (8.7) and Clinical Pharmacology (12.3) ].
2.5 Patients with Impaired Renal Function
Since there is no lower dose strength for FORFIVO XL, FORFIVO XL is not recommended in patients with renal impairment [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3) ].
3 DOSAGE FORMS AND STRENGTHS
FORFIVO XL Extended-Release Tablets, 450 mg of bupropion hydrochloride, are white to off white, oval tablets with the logo “Forfivo” printed on one side.
4 CONTRAINDICATIONS
FORFIVO XL is contraindicated in patients with the following:
- Seizure disorder because these patients may have a lower seizure threshold.
- Treated currently with other bupropion products because the incidence of seizure is dose dependent.
- Current or prior diagnosis of bulimia or anorexia nervosa because of a higher incidence of seizures noted in patients treated for bulimia with the immediate-release formulation of bupropion in a pre-marketing clinical trial.
- Undergoing abrupt discontinuation of alcohol or sedatives because of a lower seizure threshold in these conditions.
- Concurrent administration of monoamine oxidase (MAO) inhibitors because MAOIs potentially can enhance the CNS toxicity. At least 14 days should elapse between discontinuation of an MAO inhibitor and initiation of treatment with FORFIVO XL.
- Known hypersensitivity to bupropion or the other ingredients of FORFIVO XL tablets Anaphylactoid/anaphylactic reactions and Stevens-Johnson syndrome have been reported [see Warnings and Precautions (5.9) ].
5 WARNINGS AND PRECAUTIONS
5.1 Clinical Worsening and Suicide Risk in Treating Psychiatric Disorder
Patients with major depressive disorder (MDD), both adult and pediatric, may experience worsening of their depression and/or the emergence of suicidal ideation and behavior (suicidality) or unusual changes in behavior, whether or not they are taking antidepressant medications, and this risk may persist until significant remission occurs. Suicide is a known risk of depression and certain other psychiatric disorders, and these disorders themselves are the strongest predictors of suicide. There has been a long-standing concern that antidepressants may have a role in inducing worsening of depression and the emergence of suicidality in certain patients during the early phases of treatment. Pooled analyses of short-term placebo-controlled trials of antidepressant drugs (SSRIs and others) show that these drugs increase the risk of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults (ages 18-24) with major depressive disorder (MDD) and other psychiatric disorders. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction with antidepressants compared to placebo in adults aged 65 and older.
The pooled analyses of placebo-controlled trials in children and adolescents with MDD, obsessive compulsive disorder (OCD), or other psychiatric disorders included a total of 24 short-term trials of 9 antidepressant drugs in over 4,400 patients. The pooled analyses of placebo-controlled trials in adults with MDD or other psychiatric disorders included a total of 295 short-term trials (median duration of 2 months) of 11 antidepressant drugs in over 77,000 patients. There was considerable variation in risk of suicidality among drugs, but a tendency toward an increase in the younger patients for almost all drugs studied. There were differences in absolute risk of suicidality across the different indications, with the highest incidence in MDD. The risk differences (drug vs. placebo), however, were relatively stable within age strata and across indications. These risk differences (drug-placebo difference in the number of cases of suicidality per 1,000 patients treated) are provided in Table 1 .
| Age Range | |
| Increases Compared to Placebo | |
| <18 | 14 additional cases |
| 18-24 | 5 additional cases |
| Decreases Compared to Placebo | |
| 25-64 | 1 fewer case |
| ≥65 | 6 fewer cases |
No suicides occurred in any of the pediatric trials. There were suicides in the adult trials, but the number was not sufficient to reach any conclusion about drug effect on suicide.
It is unknown whether the suicidality risk extends to longer-term use, i.e., beyond several months. However, there is substantial evidence from placebo-controlled maintenance trials in adults with depression that the use of antidepressants can delay the recurrence of depression.
All patients being treated with antidepressants for any indication should be monitored appropriately and observed closely for clinical worsening, suicidality, and unusual changes in behavior, especially during the initial few months of a course of drug therapy, or at times of dose changes, either increases or decreases [see Boxed Warning and Use in Specific Populations (8.4) ].
The following symptoms, anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania, and mania, have been reported in adult and pediatric patients being treated with antidepressants for major depressive disorder as well as for other indications, both psychiatric and nonpsychiatric. Although a causal link between the emergence of such symptoms and either the worsening of depression and/or the emergence of suicidal impulses has not been established, there is concern that such symptoms may represent precursors to emerging suicidality.
Consideration should be given to changing the therapeutic regimen, including possibly discontinuing the medication, in patients whose depression is persistently worse, or who are experiencing emergent suicidality or symptoms that might be precursors to worsening depression or suicidality, especially if these symptoms are severe, abrupt in onset, or were not part of the patient’s presenting symptoms.
Families and caregivers of patients being treated with antidepressants for major depressive disorder or other indications, both psychiatric and nonpsychiatric, should be alerted about the need to monitor patients for the emergence of agitation, irritability, unusual changes in behavior, and the other symptoms described above, as well as the emergence of suicidality, and to report such symptoms immediately to health care providers. Such monitoring should include daily observation by families and caregivers [see Patient Counseling Information (17) ]. Prescription for FORFIVO XL should be written for the smallest quantity of tablets consistent with good patient management, in order to reduce the risk of overdose.
5.2 Neuropsychiatric Symptoms and Suicide Risk in Smoking Cessation Treatment
FORFIVO XL is not approved for smoking cessation treatment, but bupropion under the name ZYBAN is approved for this use. Serious neuropsychiatric symptoms have been reported in patients taking bupropion for smoking cessation [see Boxed Warning and Adverse Reactions (6) ]. These have included changes in mood (including depression and mania), psychosis, hallucinations, paranoia, delusions, homicidal ideation, hostility, agitation, aggression, anxiety, and panic, as well as suicidal ideation, suicide attempt, and completed suicide [see Patient Counseling Information (17) ].
5.3 Activation of Mania/Hypomania
A major depressive episode may be the initial presentation of bipolar disorder. It is generally believed (though not established in controlled trials) that treating such an episode with an antidepressant alone may increase the likelihood of precipitation of a mixed/manic episode in patients at risk for bipolar disorder. Prior to initiating treatment with an antidepressant, patients with depressive symptoms should be adequately screened to determine if they are at risk for bipolar disorder; such screening should include a detailed psychiatric history, including a family history of suicide, bipolar disorder, and depression. It should be noted that FORFIVO XL is not approved for use in treating bipolar depression.
5.4 Seizures
Bupropion is associated with a dose-related risk of seizures. The risk of seizures is also related to patient factors, clinical situations, and concomitant medications, which must be considered in selection of patients for therapy with FORFIVO XL. FORFIVO XL should be discontinued and not restarted in patients who experience a seizure while on treatment.
Dose
At doses up to 300 mg/day of the sustained-release formulation of bupropion hydrochloride (WELLBUTRIN SR®), the incidence of seizure is approximately 0.1% (1/1,000).
Data for the immediate-release formulation of bupropion hydrochloride revealed a seizure incidence of approximately 0.4% (i.e., 13 of 3,200 patients followed prospectively) in patients treated at doses in a range of 300 to 450 mg/day. This seizure incidence (0.4%) may exceed that of some other marketed antidepressants.
Additional data accumulated for the immediate-release formulation of bupropion hydrochloride suggested that the estimated seizure incidence increases almost tenfold between 450 and 600 mg/day. The 600 mg dose is twice the usual adult dose and one and one-third the maximum recommended daily dose (450 mg) of FORFIVO XL. This disproportionate increase in seizure incidence with dose incrementation calls for caution in dosing.
Patient Factors
Predisposing factors that may increase the risk of seizure with bupropion use include history of head trauma or prior seizure, central nervous system (CNS) tumor, the presence of severe hepatic cirrhosis, and concomitant medications that lower seizure threshold.
Clinical Situations
Circumstances associated with an increased seizure risk include, among others, excessive use of alcohol or sedatives; addiction to opiates, cocaine, or stimulants; use of over-the-counter stimulants and anorectics; and diabetes treated with oral hypoglycemics or insulin.
Concomitant Medications
Many medications (e.g., antipsychotics, antidepressants, theophylline, and systemic steroids) are known to lower seizure threshold.
Recommendations for Reducing the Risk of Seizure:
Retrospective analysis of clinical experience gained during the development of bupropion suggests that the risk of seizure may be minimized if
- the total daily dose of bupropion does not exceed 450 mg,
- the rate of incrementation of the bupropion dose is gradual.
5.5 Psychosis and Other Neuropsychiatric Events
Depressed patients treated with bupropion have been reported to show a variety of neuropsychiatric signs and symptoms, including delusions, hallucinations, psychosis, concentration disturbance, paranoia, and confusion. In some cases, these symptoms abated upon dose reduction and/or withdrawal of treatment. It is recommended stopping bupropion when the symptoms occurred.
5.6 Severe Hypertension
In clinical practice, hypertension, in some cases severe, requiring acute treatment, has been reported in patients receiving bupropion alone and in combination with nicotine replacement therapy. These reactions have been observed in both patients with and without evidence of preexisting hypertension.
Data from a comparative study of the sustained-release formulation of bupropion hydrochloride (ZYBAN® Sustained-Release Tablets), nicotine transdermal system (NTS), the combination of sustained-release bupropion hydrochloride plus NTS, and placebo as an aid to smoking cessation suggest a higher incidence of treatment-emergent hypertension in patients treated with the combination of sustained-release bupropion hydrochloride and NTS. Monitoring of blood pressure is recommended in patients who receive the combination of bupropion and nicotine replacement.
There is no clinical experience establishing the safety of FORFIVO XL tablets in patients with a recent history of myocardial infarction or unstable heart disease. Therefore, care should be exercised if it is used in these groups.
Bupropion was well tolerated in depressed patients who had previously developed orthostatic hypotension while receiving tricyclic antidepressants, and was also generally well tolerated in a group of 36 depressed inpatients with stable congestive heart failure (CHF). However, bupropion was associated with a rise in supine blood pressure in the study of patients with CHF, resulting in discontinuation of treatment in 2 patients for exacerbation of baseline hypertension.
5.7 Agitation and Insomnia
Increased restlessness, agitation, anxiety, and insomnia, especially shortly after initiation of treatment, have been associated with treatment with bupropion.
Patients in placebo-controlled trials of major depressive disorder with sustained-release formulation of bupropion hydrochloride, experienced agitation, anxiety, and insomnia as shown in Table 2 .
|
Adverse Reactions Term |
Bupropion HCl 300 mg/day (n = 376) |
Bupropion HCl 400 mg/day (n = 114) |
Placebo (n = 385) |
| Agitation | 3% | 9% | 2% |
| Anxiety | 5% | 6% | 3% |
| Insomnia | 11% | 16% | 6% |
In clinical studies of major depressive disorder, these symptoms were sometimes of sufficient magnitude to require treatment with sedative/hypnotic drugs.
Symptoms in these studies were sufficiently severe to require discontinuation of treatment in 1% and 2.6% of patients treated with 300 and 400 mg/day, respectively, of bupropion hydrochloride sustained-release tablets and 0.8% of patients treated with placebo.
5.8 Altered Appetite and Weight
In placebo-controlled short-term studies of major depressive disorder using the sustained-release formulation of bupropion hydrochloride, patients experienced weight gain or weight loss as shown in Table 3 .
| Weight Change |
Bupropion HCl 300 mg/day (n = 339) |
Bupropion HCl 400 mg/day (n = 112) |
Placebo (n = 347) |
| Gained >5 lbs | 3% | 2% | 4% |
| Lost >5 lbs | 14% | 19% | 6% |
In studies conducted with the immediate-release formulation of bupropion hydrochloride, 35% of patients receiving tricyclic antidepressants gained weight, compared to 9% of patients treated with the immediate-release formulation of bupropion hydrochloride. If weight loss is a major presenting sign of a patient’s depressive illness, the anorectic and/or weight-reducing potential of FORFIVO XL tablets should be considered.
5.9 Hypersensitivity Reactions
Anaphylactoid/anaphylactic reactions characterized by symptoms such as pruritus, urticaria, angioedema, and dyspnea requiring medical treatment have been reported in clinical trials with bupropion. In addition, there have been rare spontaneous postmarketing reports of erythema multiforme, Stevens-Johnson syndrome, and anaphylactic shock associated with bupropion. A patient should stop taking FORFIVO XL and consult a doctor if experiencing allergic or anaphylactoid/anaphylactic reactions (e.g., skin rash, pruritus, hives, chest pain, edema, and shortness of breath) during treatment.
Arthralgia, myalgia, and fever with rash and other symptoms suggestive of delayed hypersensitivity have been reported in association with bupropion. These symptoms may resemble serum sickness [see Contraindications (4) ].
6 ADVERSE REACTIONS
The following risks are discussed in greater detail in other sections of the labeling:
- Clinical worsening and suicide risk [see Warnings and Precautions (5.1) ]
- Neuropsychiatric symptoms and suicide risk in smoking cessation treatment [see Warnings and Precautions (5.2) ]
- Activation of mania or hypomania [see Warnings and Precautions (5.3) ]
- Seizures [see Warnings and Precautions (5.4) ]
- Psychosis, and other neuropsychiatric events [see Warnings and Precautions (5.5) ]
- Severe hypertension [see Warnings and Precautions (5.6) ]
- Agitation and insomnia [see Warnings and Precautions (5.7) ]
- Altered appetite and weight [see Warnings and Precautions (5.8) ]
- Hypersensitivity reactions [see Warnings and Precautions (5.9) ]
6.1 Clinical Trials Experience
Commonly Observed Adverse Reactions in Controlled Clinical Trials
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Adverse reactions from Table 5 occurring in at least 5% of patients treated with the sustained-release formulation of bupropion hydrochloride and at a rate at least twice the placebo rate are listed below for the 300- and 400-mg/day dose groups.
300 mg/day of bupropion sustained release: Anorexia, dry mouth, rash, sweating, tinnitus, and tremor.
400 mg/day of bupropion sustained release: Abdominal pain, agitation, anxiety, dizziness, dry mouth, insomnia, myalgia, nausea, palpitation, pharyngitis, sweating, tinnitus, and urinary frequency.
FORFIVO XL is bioequivalent to three 150 mg tablets of WELLBUTRIN XL®, which has been demonstrated to have similar bioavailability both to the immediate-release formulation of bupropion and to the sustained-release formulation of bupropion. The information included under this subsection and under subsections 6.2 and 6.3 is based primarily on data from controlled clinical trials with the sustained-release formulation of bupropion hydrochloride.
Adverse Reactions Leading to Discontinuation of Treatment with Bupropion Immediate Release or Bupropion Sustained Release
In placebo-controlled clinical trials, 9% and 11% of patients treated with 300 and 400 mg/day, respectively, of the sustained-release formulation of bupropion hydrochloride and 4% of patients treated with placebo discontinued treatment due to adverse reactions. The specific adverse reactions in these trials that led to discontinuation in at least 1% of patients treated with either 300 mg/day or 400 mg/day of the sustained-release formulation of bupropion hydrochloride, and at a rate at least twice the placebo rate are listed in Table 4 .
| Adverse Reaction |
Bupropion HCl 300 mg/day (n = 376) |
Bupropion HCl 400 mg/day (n = 114) |
Placebo (n = 385) |
| Rash | 2.4% | 0.9% | 0.0% |
| Nausea | 0.8% | 1.8% | 0.3% |
| Agitation | 0.3% | 1.8% | 0.3% |
| Migraine | 0.0% | 1.8% | 0.3% |
In clinical trials with the immediate-release formulation of bupropion, 10% of patients and volunteers discontinued due to an adverse reaction. Reactions resulting in discontinuation, in addition to those listed above for the sustained-release formulation of bupropion hydrochloride, include vomiting, seizures, and sleep disturbances.
Adverse Reactions Occurring at an Incidence of 1% or More Among Patients Treated With Bupropion Immediate Release or Bupropion Sustained Release
Table 5 enumerates adverse reactions that occurred among patients treated with 300 and 400 mg/day of the sustained-release formulation of bupropion hydrochloride and with placebo in controlled trials. Reactions that occurred in either the 300- or 400-mg/day group at an incidence of 1% or more and were more frequent than in the placebo group are included. Reported adverse reactions were classified using a COSTART-based Dictionary.
Accurate estimates of the incidence of adverse reactions associated with the use of any drug are difficult to obtain. Estimates are influenced by drug dose, detection technique, setting, physician judgments, etc. The figures cited cannot be used to predict precisely the incidence of untoward reactions in the course of usual medical practice where patient characteristics and other factors differ from those that prevailed in the clinical trials. These incidence figures also cannot be compared with those obtained from other clinical studies involving related drug products as each group of drug trials is conducted under a different set of conditions.
Finally, it is important to emphasize that the tabulation does not reflect the relative severity and/or clinical importance of the reactions. A better perspective on the serious adverse reactions associated with the use of bupropion is provided in the Warnings and Precautions (5) .
| — Hyphen denotes adverse reactions occurring in greater than 0 but less than 0.5% of patients. | |||
| Body System/Adverse Reaction |
Bupropion HCl 300 mg/day (n = 376) |
Bupropion HCl 400 mg/day (n = 114) |
Placebo (n = 385) |
| Body (General) | |||
| Headache | 26% | 25% | 23% |
| Infection | 8% | 9% | 6% |
| Abdominal pain | 3% | 9% | 2% |
| Asthenia | 2% | 4% | 2% |
| Chest pain | 3% | 4% | 1% |
| Pain | 2% | 3% | 2% |
| Fever | 1% | 2% | — |
| Cardiovascular | |||
| Palpitation | 2% | 6% | 2% |
| Flushing | 1% | 4% | — |
| Migraine | 1% | 4% | 1% |
| Hot flashes | 1% | 3% | 1% |
| Digestive | |||
| Dry mouth | 17% | 24% | 7% |
| Nausea | 13% | 18% | 8% |
| Constipation | 10% | 5% | 7% |
| Diarrhea | 5% | 7% | 6% |
| Anorexia | 5% | 3% | 2% |
| Vomiting | 4% | 2% | 2% |
| Dysphagia | 0% | 2% | 0% |
| Musculoskeletal | |||
| Myalgia | 2% | 6% | 3% |
| Arthralgia | 1% | 4% | 1% |
| Arthritis | 0% | 2% | 0% |
| Twitch | 1% | 2% | — |
| Nervous system | |||
| Insomnia | 11% | 16% | 6% |
| Dizziness | 7% | 11% | 5% |
| Agitation | 3% | 9% | 2% |
| Anxiety | 5% | 6% | 3% |
| Tremor | 6% | 3% | 1% |
| Nervousness | 5% | 3% | 3% |
| Somnolence | 2% | 3% | 2% |
| Irritability | 3% | 2% | 2% |
| Memory decreased | — | 3% | 1% |
| Paresthesia | 1% | 2% | 1% |
| Central nervous system stimulation | 2% | 1% | 1% |
| Respiratory | |||
| Pharyngitis | 3% | 11% | 2% |
| Sinusitis | 3% | 1% | 2% |
| Increased cough | 1% | 2% | 1% |
| Skin | |||
| Sweating | 6% | 5% | 2% |
| Rash | 5% | 4% | 1% |
| Pruritus | 2% | 4% | 2% |
| Urticaria | 2% | 1% | 0% |
| Special senses | |||
| Tinnitus | 6% | 6% | 2% |
| Taste perversion | 2% | 4% | — |
| Blurred vision or diplopia | 3% | 2% | 2% |
| Urogenital | |||
| Urinary frequency | 2% | 5% | 2% |
| Urinary urgency | — | 2% | 0% |
Vaginal hemorrhage |
0% | 2% | — |
| Urinary tract infection | 1% | 0% | — |
Additional reactions to those listed in Table 5 that occurred at an incidence of at least 1% in controlled clinical trials of the immediate-release formulation of bupropion hydrochloride (300 to 600 mg/day) and that were numerically more frequent than placebo were: cardiac arrhythmias (5% vs. 4%), hypertension (4% vs. 2%), hypotension (3% vs. 2%), tachycardia (11% vs. 9%), appetite increase (4% vs. 2%), dyspepsia (3% vs. 2%), menstrual complaints (5% vs. 1%), akathisia (2% vs. 1%), impaired sleep quality (4% vs. 2%), sensory disturbance (4% vs. 3%), confusion (8% vs. 5%), decreased libido (3% vs. 2%), hostility (6% vs. 4%), auditory disturbance (5% vs. 3%), and gustatory disturbance (3% vs. 1%).
Other adverse reactions occurring < 1% in clinical trials:
Chills, facial edema, postural hypotension, stroke, syncope, bruxism, gastric reflux, gingivitis, glossitis, increased salivation, mouth ulcers, stomatitis, edema of tongue, ecchymosis, edema, abnormal coordination, decreased libido, depersonalization, emotional lability, hyperkinesia, hypertonia, hypesthesia, ataxia, and derealization, bronchospasm, accommodation abnormality, dry eye, impotence, and prostate disorder.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of Bupropion hydrochloride. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Only those adverse reactions not previously listed for bupropion are included. The extent to which these reactions may be associated with FORFIVO XL is unknown.
Cardiovascular—complete atrioventricular block, extrasystoles, myocardial infarction, phlebitis, and pulmonary embolism.
Gastrointestinal—colitis, esophagitis, gastrointestinal hemorrhage, gum hemorrhage, intestinal perforation, pancreatitis, and stomach ulcer.
Endocrine—hyperglycemia, hypoglycemia, and syndrome of inappropriate antidiuretic hormone.
Hemic and Lymphatic—anemia, leukocytosis, leukopenia, lymphadenopathy, pancytopenia, and thrombocytopenia.
Metabolic and Nutritional—glycosuria.
Musculoskeletal—muscle rigidity/fever/rhabdomyolysis and muscle weakness.
Nervous System—abnormal electroencephalogram (EEG), aggression, akinesia, aphasia, coma, delirium,-dysarthria, dyskinesia, dystonia, extrapyramidal syndrome, hypokinesia, increased libido, neuralgia, neuropathy, and unmasking tardive dyskinesia.
Skin—alopecia, exfoliative dermatitis, and hirsutism.
Eye—mydriasis.
Urogenital—abnormal ejaculation, cystitis, dyspareunia, dysuria, gynecomastia, painful erection, salpingitis, urinary incontinence, and urinary retention.
7 DRUG INTERACTIONS
Few systemic data have been collected on the metabolism of bupropion following concomitant administration with other drugs or, alternatively, the effect of concomitant administration of bupropion on the metabolism of other drugs.
7.1 Potential for Other Drugs to Affect FORFIVO XL
Because bupropion is extensively metabolized, the coadministration of other drugs may affect its clinical activity.
Substrates or Inhibitors/Inducers of Cytochrome P450IIB6 (CYP2B6): In vitro studies indicate that bupropion is primarily metabolized to hydroxybupropion by the CYP2B6 isoenzyme. Therefore, the potential exists for a drug interaction between FORFIVO XL and drugs that are substrates or inhibitors/inducers of the CYP2B6 isoenzyme (e.g., orphenadrine, thiotepa, cyclophosphamide, ticlopidine and clopidogrel). In addition, in vitro studies suggest that paroxetine, sertraline, norfluoxetine, and fluvoxamine as well as nelfinavir, inhibit the hydroxylation of bupropion.
-
-
-
Cimetidine: The threohydrobupropion metabolite of bupropion does not appear to be produced by the cytochrome P450 isoenzymes. The effects of concomitant administration of cimetidine on the pharmacokinetics of bupropion and its active metabolites were studied in 24 healthy young male volunteers. Following oral administration of two 150-mg tablets of the sustained-release formulation of bupropion hydrochloride with and without 800 mg of cimetidine, the pharmacokinetics of bupropion and hydroxybupropion were unaffected. However, there were 16% and 32% increases in the AUC and Cmax, respectively, of the combined moieties of threohydrobupropion and erythrohydrobupropion.
Carbamazepine, Phenobarbital, Phenytoin: While not systematically studied, these drugs may induce the metabolism of bupropion.
7.2 Potential for FORFIVO XL to Affect Other Drugs
Animal data indicated that bupropion may be an inducer of drug-metabolizing enzymes in humans. In one study, following chronic administration of bupropion hydrochloride, 100 mg 3 times daily to 8 healthy male volunteers for 14 days, there was no evidence of induction of its own metabolism. Nevertheless, there may be the potential for clinically important alterations of blood levels of coadministered drugs.
Lamotrigine: Multiple oral doses of bupropion had no statistically significant effects on the single dose pharmacokinetics of lamotrigine in 12 healthy volunteers.
Drugs Metabolized by Cytochrome P450IID6 (CYP2D6)
Many drugs, including most antidepressants (SSRIs, many tricyclics), beta-blockers, antiarrhythmics, and antipsychotics are metabolized by the CYP2D6 isoenzyme. Although bupropion is not metabolized by this isoenzyme, bupropion and hydroxybupropion are inhibitors of the CYP2D6 isoenzyme in vitro. In a study of 15 male subjects (ages 19 to 35 years) who were extensive metabolizers of the CYP2D6 isoenzyme, daily doses of bupropion hydrochloride given as 150 mg twice daily followed by a single dose of 50 mg desipramine increased the Cmax, AUC, and t1/2 of desipramine by an average of approximately 2-, 5-, and 2-fold, respectively. The effect was present for at least 7 days after the last dose of bupropion. Concomitant use of bupropion with other drugs metabolized by CYP2D6 has not been formally studied.
Therefore, coadministration of bupropion with drugs that are metabolized by the CYP2D6 isoenzyme including certain antidepressants (e.g., venlafaxine, nortriptyline, imipramine, desipramine, paroxetine, fluoxetine, and sertraline), antipsychotics (e.g., haloperidol, risperidone, and thioridazine), beta-blockers (e.g., metoprolol), and Type 1C antiarrhythmics (e.g., propafenone, and flecainide), should be approached with caution and should be initiated at the lower end of the dose range of the concomitant medication. If bupropion is added to the treatment regimen of a patient already receiving a drug metabolized by CYP2D6, the need to decrease the dose of the original medication should be considered, particularly for those concomitant medications with a narrow therapeutic index.
CYP2D6 in order to be effective (e.g., tamoxifen) theoretically could have reduced efficacy when administered concomitantly with inhibitors of CYP2D6 such as bupropion.
Although citalopram is not primarily metabolized by CYP2D6, in one study bupropion increased the Cmax and AUC of citalopram by 30% and 40%, respectively. Citalopram did not affect the pharmacokinetics of bupropion and its three metabolites.
7.3 Nicotine Transdermal System
Data from a smoking cessation study suggest that a higher incidence of hypertension in patients who received the combination of sustained-release bupropion hydrochloride and nicotine transdermal system (NTS). Monitoring of blood pressure is recommended in patients who receive the combination of bupropion and nicotine replacement [see Warnings and Precautions (5.6) ].
7.4 Drug Laboratory Test Interactions
False-positive urine immunoassay screening tests for amphetamines have been reported in patients taking bupropion. This is due to lack of specificity of some screening tests. False-positive test results may result even following discontinuation of bupropion therapy. Confirmatory test such as gas chromatography/mass spectrometry, will distinguish bupropion from amphetamines.
7.5 MAO Inhibitors
Studies in animals demonstrate that the acute toxicity of bupropion is enhanced by the MAO inhibitor phenelzine [see Contraindications (4) ].
7.6 Drugs that Lower Seizure Threshold
Since there is no lower strength for FORFIVO XL, concurrent administration of FORFIVO XL tablets and agents (e.g., antipsychotics, other antidepressants, theophylline, systemic steroids, etc.) that lower seizure threshold should be undertaken only with caution [see Warnings and Precautions (5.4) ].
7.7 Alcohol
In postmarketing experience, there have been rare reports of adverse neuropsychiatric events or reduced alcohol tolerance in patients who were drinking alcohol during treatment with bupropion. Alcohol increased the release rate of FORFIVO XL in vitro. The consumption of alcohol during treatment with FORFIVO XL should be avoided.
7.8 Levodopa and Amantadine
Limited clinical data suggest a higher incidence of adverse experiences in patients receiving bupropion concurrently with either levodopa or amantadine. Since there is no lower strength for FORFIVO XL, administration of FORFIVO XL tablets to patients receiving either levodopa or amantadine concurrently should be undertaken with caution.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C. In studies conducted in rats and rabbits, bupropion hydrochloride was administered orally at doses up to 450 and 150 mg/kg/day, respectively (approximately 11 and 7 times the maximum recommended human dose [MRHD], respectively, on a mg/m2 basis), during the period of organogenesis. No clear evidence of teratogenic activity was found in either species; however, in rabbits, slightly increased incidences of fetal malformations and skeletal variations were observed at the lowest dose tested (25 mg/kg/day, approximately equal to the MRHD on a mg/m2 basis) and greater. Decreased fetal weights were seen at 50 mg/kg and greater.
When rats were administered bupropion hydrochloride at oral doses of up to 300 mg/kg/day (approximately 7 times the MRHD on a mg/m2 basis) prior to mating and throughout pregnancy and lactation, there were no apparent adverse effects on offspring development.
One study has been conducted in pregnant women. This retrospective, managed-care database study assessed the risk of congenital malformations overall, and cardiovascular malformations specifically, following exposure to bupropion in the first trimester compared to the risk of these malformations following exposure to other antidepressants in the first trimester and bupropion outside of the first trimester. This study included 7,005 infants with antidepressant exposure during pregnancy, 1,213 of whom were exposed to bupropion in the first trimester. The study showed no greater risk for congenital malformations overall, or cardiovascular malformations specifically, following first trimester bupropion exposure compared to exposure to all other antidepressants in the first trimester, or bupropion outside of the first trimester. The results of this study have not been corroborated. FORFIVO XL should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
8.3 Nursing Mothers
Like many other drugs, bupropion and its metabolites are secreted in human milk. Because of the potential for serious adverse reactions in nursing infants from FORFIVO XL tablets, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
8.4 Pediatric Use
Safety and effectiveness in pediatric patients have not been established. Anyone considering the use of FORFIVO XL in a child or adolescent must balance the potential risks with the clinical need.
8.5 Geriatric Use
Of the approximately 6,000 patients who participated in clinical trials with bupropion hydrochloride sustained-release tablets (depression and smoking cessation studies), 275 were 65 years old and over and 47 were 75 years old and over. In addition, several hundred patients 65 and over participated in clinical trials using the immediate-release formulation of bupropion hydrochloride (depression studies). No overall differences in safety or effectiveness were observed between these subjects and younger subjects. Reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
A single-dose pharmacokinetic study demonstrated that the disposition of bupropion and its metabolites in elderly subjects was similar to that of younger subjects; however, another pharmacokinetic study, single and multiple dose, has suggested that the elderly are at increased risk for accumulation of bupropion and its metabolites [see Clinical Pharmacology (12.3) ].
Bupropion is extensively metabolized in the liver to active metabolites, which are further metabolized and excreted by the kidneys. The risk of toxic reaction to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function [see Dosage and Administration (2.5) and Use in Specific Populations (8.6) ].
8.6 Renal Impairment
Since there is no lower dose strength for FORFIVO XL, FORFIVO XL is not recommended in patients with renal impairment [see Clinical Pharmacology (12.3) ].
8.7 Hepatic Impairment
Since there is no lower dose strength for FORFIVO XL, FORFIVO XL is not recommended in patients with hepatic impairment [see Clinical Pharmacology (12.3) ].
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
Bupropion is not a controlled substance.
9.2 Abuse
Humans
Controlled clinical studies of bupropion hydrochloride (immediate-release formulation) conducted in normal volunteers, in subjects with a history of multiple drug abuse, and in depressed patients showed some increase in motor activity and agitation/excitement.
In a population of individuals experienced with drugs of abuse, a single dose of 400 mg of bupropion hydrochloride produced mild amphetamine-like activity as compared to placebo on the Morphine-Benzedrine Subscale of the Addiction Research Center Inventories (ARCI), and a score intermediate between placebo and amphetamine on the Liking Scale of the ARCI. These scales measure general feelings of euphoria and drug desirability.
Findings in clinical trials, however, are not known to reliably predict the abuse potential of drugs. Nonetheless, evidence from single-dose studies does suggest that the recommended daily dosage of bupropion when administered in divided doses is not likely to be especially reinforcing to amphetamine or stimulant abusers. However, higher doses that could not be tested because of the risk of seizure might be modestly attractive to those who abuse stimulant drugs.
Animals
Studies in rodents and primates have shown that bupropion exhibits some pharmacologic actions common to psychostimulants. In rodents, it has been shown to increase locomotor activity, elicit a mild stereotyped behavioral response, and increase rates of responding in several schedule-controlled behavior paradigms. In primate models to assess the positive reinforcing effects of psychoactive drugs, bupropion was self-administered intravenously. In rats, bupropion produced amphetamine-like and cocaine-like discriminative stimulus effects in drug discrimination paradigms used to characterize the subjective effects of psychoactive drugs.
10 OVERDOSAGE
10.1 Human Overdose Experience
Overdoses of up to 30 g or more of bupropion have been reported. Seizure was reported in approximately one third of all cases. Other serious reactions reported with overdoses of bupropion alone included hallucinations, loss of consciousness, sinus tachycardia, and ECG changes such as conduction disturbances or arrhythmias. Fever, muscle rigidity, rhabdomyolysis, hypotension, stupor, coma, and respiratory failure have been reported mainly when bupropion was part of multiple drug overdoses.
Although most patients recovered without sequelae, deaths associated with overdoses of bupropion alone have been reported in patients ingesting large doses of the drug. Multiple uncontrolled seizures, bradycardia, cardiac failure, and cardiac arrest prior to death were reported in these patients.
10.2 Overdosage Management
Ensure an adequate airway, oxygenation, and ventilation. Monitor cardiac rhythm and vital signs. EEG monitoring is also recommended for the first 48 hours post-ingestion. General supportive and symptomatic measures are also recommended. Induction of emesis is not recommended. Gastric lavage with a large-bore orogastric tube with appropriate airway protection, if needed, may be indicated if performed soon after ingestion or in symptomatic patients.
Activated charcoal should be administered. There is no experience with the use of forced diuresis, dialysis, hemoperfusion, or exchange transfusion in the management of bupropion overdoses. No specific antidotes for bupropion are known.
Due to the dose-related risk of seizures with FORFIVO XL, hospitalization following suspected overdose should be considered. Based on studies in animals, it is recommended that seizures be treated with intravenous benzodiazepine administration and other supportive measures, as appropriate.
In managing overdosage, consider the possibility of multiple drug involvement. The physician should consider contacting a poison control center for additional information on the treatment of any overdose. Telephone numbers for certified poison control centers are listed in the Physicians’ Desk Reference (PDR).
11 DESCRIPTION
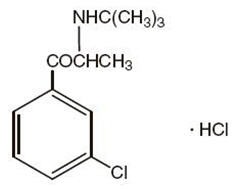
FORFIVO XL (bupropion hydrochloride), an antidepressant of the aminoketone class, is chemically unrelated to tricyclic, tetracyclic, selective serotonin re-uptake inhibitor, or other known antidepressant agents. Its structure closely resembles that of diethylpropion; it is related to phenylethylamines. It is designated as (±)-2-(tert-Butylamino)-3'-chloropropiophenone hydrochloride. The molecular weight is 276.2. The empirical formula is C13H18ClNO·HCl. Bupropion hydrochloride powder is white or almost white, crystalline, and soluble in water. It has a bitter taste and produces the sensation of local anesthesia on the oral mucosa. The structural formula is:
FORFIVO XL tablets are supplied for oral administration of 450 mg of bupropion hydrochloride as white to off white extended-release tablets. Each film coated tablet contains the labeled amount of bupropion hydrochloride and the inactive ingredients: hydroxypropyl cellulose, hydrochloric acid, polyvinyl pyrrolidone and polyvinyl acetate blend, polyethylene oxide, stearic acid, colloidal silicon dioxide, magnesium stearate, hydroxypropylmethyl cellulose, triacetin, talc, methacrylic acid copolymer, polyethylene glycol 8000, titanium dioxide and carboxymethyl cellulose sodium. The logo “Forfivo” is printed on one side of the tablet with edible black ink.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of action of bupropion is unknown, as is the case with other antidepressants. However, it is presumed that this action is mediated by noradrenergic and/or dopaminergic mechanisms.
12.2 Pharmacodynamics
Bupropion is a relatively weak inhibitor of the neuronal uptake of norepinephrine and dopamine, and does not inhibit monoamine oxidase or the re-uptake of serotonin.
12.3 Pharmacokinetics
Bupropion is a racemic mixture. The pharmacologic activity and pharmacokinetics of the individual enantiomers have not been studied.
Following single dosing under fasted conditions of FORFIVO XL tablets, the maximum peak plasma concentration (Cmax), and the area under the plasma concentration vs. time curve of bupropion from zero to infinity (AUCinf), were 207.46 (±59.40) ng/mL, and 2147.53 (±664.12) ng•hr/mL, respectively. The elimination half-life (±SD) of bupropion after a single dose was 14.44 (±5.00) hours.
In a single dose study under fasting conditions, one FORFIVO XL tablet given once daily and three Wellbutrin XL® 150 mg tablets once daily were evaluated. Equivalence was demonstrated for peak concentration and area under the curve for bupropion and the 3 metabolites (hydroxybupropion, erythrhydrobupropion, and threohydrobupropion).
Absorption
Following single oral administration of FORFIVO XL tablets to healthy volunteers, the median time to peak plasma concentrations for bupropion was approximately 5 hours under fasted conditions, and 12 hours under fed conditions. The presence of food did not affect the maximum peak plasma concentration for bupropion, however, mean systemic exposure to bupropion was increased by 25% when FORFIVO XL tablet was taken with food. The food effect is not considered clinically significant and FORFIVO XL can be taken with or without food.
Distribution
In vitro tests show that bupropion is 84% bound to human plasma proteins at concentrations up to 200 mcg/mL. The extent of protein binding of the hydroxybupropion metabolite is similar to that for bupropion, whereas the extent of protein binding of the threohydrobupropion metabolite is about half that seen with bupropion.
Metabolism
Bupropion is extensively metabolized in humans. Three metabolites have been shown to be active: hydroxybupropion, which is formed via hydroxylation of the tert-butyl group of bupropion, and the amino-alcohol isomers threohydrobupropion and erythrohydrobupropion, which are formed via reduction of the carbonyl group. In vitro findings suggest that cytochrome P450IIB6 (CYP2B6) is the principal isoenzyme involved in the formation of hydroxybupropion, while cytochrome P450 isoenzymes are not involved in the formation of threohydrobupropion. Oxidation of the bupropion side chain results in the formation of a glycine conjugate of metachlorobenzoic acid, which is then excreted as the major urinary metabolite. The potency and toxicity of the metabolites relative to bupropion have not been fully characterized. However, it has been demonstrated in an antidepressant screening test in mice that hydroxybupropion is one half as potent as bupropion, while threohydrobupropion and erythrohydrobupropion are 5-fold less potent than bupropion. This may be of clinical importance because the plasma concentrations of the metabolites are as high or higher than those of bupropion.
Because bupropion is extensively metabolized, there is the potential for drug-drug interactions, particularly with those agents that are metabolized by the cytochrome P450IIB6 (CYP2B6) isoenzyme, such as ritonavir or efavirenz. In a healthy volunteer study, ritonavir at a dose of 100 mg twice daily reduced the AUC and Cmax of bupropion by 22% and 21%, respectively. The exposure of the hydroxybupropion metabolite was decreased by 23%, the threohydrobupropion metabolite decreased by 38% and the erythrohydrobupropion metabolite decreased by 48%.
In a second healthy volunteer study, ritonavir at a dose of 600 mg twice daily decreased the AUC and the Cmax of bupropion by 66% and 62%, respectively. The exposure of the hydroxybupropion metabolite was decreased by 78%, the threohydrobupropion decreased by 50% and the erythrohydrobupropion decreased by 68%.
In another healthy volunteer study, KALETRA® (lopinavir 400 mg/ritonavir 100 mg twice daily) decreased bupropion AUC and Cmax by 57%. The AUC and Cmax of hydroxybupropion were decreased by 50% and 31%, respectively [see Drug Interactions (7) ].
In a study in healthy volunteers, efavirenz 600 mg once daily for 2 weeks reduces the AUC and Cmax of bupropion by approximately 55% and 34%, respectively. The AUC of hydroxybupropion was unchanged, whereas Cmax of hydroxybupropion was increased by 50%.
Although bupropion is not metabolized by cytochrome P450IID6 (CYP2D6), there is the potential for drug-drug interactions when bupropion is coadministered with drugs metabolized by this isoenzyme [see Drug Interactions (7.2) ].
In humans, peak plasma concentrations of hydroxybupropion occur approximately 10 hours after administration of single dose of FORFIVO XL under fasted conditions and 16 hours under fed conditions. Following administration of WELLBUTRIN XL, peak plasma concentrations of hydroxybupropion are approximately 7 times the peak level of the parent drug at steady state. The elimination half-life of hydroxybupropion is approximately 20 (±5) hours, and its AUC at steady state is about 13 times that of bupropion. The times to peak concentrations for the erythrohydrobupropion and threohydrobupropion metabolites are similar to that of the hydroxybupropion metabolite. However, their elimination half-lives are longer, approximately 33 (±10) and 37 (±13) hours, respectively, and steady-state AUCs are 1.4 and 7 times that of bupropion, respectively.
Bupropion and its metabolites exhibit linear kinetics following chronic administration of 300 to 450 mg/day of bupropion hydrochloride.
Elimination
Following oral administration of 200 mg of 14C-bupropion in humans, 87% and 10% of the radioactive dose were recovered in the urine and feces, respectively. However, the fraction of the oral dose of bupropion excreted unchanged was only 0.5%, a finding consistent with the extensive metabolism of bupropion.
Population Subgroups
Factors or conditions altering metabolic capacity (e.g., liver disease, congestive heart failure [CHF], age, concomitant medications, etc.) or elimination may be expected to influence the degree and extent of accumulation of the active metabolites of bupropion. The elimination of the major metabolites of bupropion may be affected by reduced renal or hepatic function because they are moderately polar compounds and are likely to undergo further metabolism or conjugation in the liver prior to urinary excretion.
Hepatic
The effect of hepatic impairment on the pharmacokinetics of bupropion was characterized in 2 single-dose studies, one in patients with alcoholic liver disease and one in patients with mild to severe cirrhosis. The first study showed that the half-life of hydroxybupropion was significantly longer in 8 patients with alcoholic liver disease than in 8 healthy volunteers (32±14 hours versus 21±5 hours, respectively). Although not statistically significant, the AUCs for bupropion and hydroxybupropion were more variable and tended to be greater (by 53% to 57%) in patients with alcoholic liver disease. The differences in half-life for bupropion and the other metabolites in the 2 patient groups were minimal.
The second study showed no statistically significant differences in the pharmacokinetics of bupropion and its active metabolites in 9 patients with mild to moderate hepatic cirrhosis compared to 8 healthy volunteers. However, more variability was observed in some of the pharmacokinetic parameters for bupropion (AUC, Cmax, and Tmax) and its active metabolites (t½) in patients with mild to moderate hepatic cirrhosis. In addition, in patients with severe hepatic cirrhosis, the bupropion Cmax and AUC were substantially increased (mean difference: by approximately 70% and 3-fold, respectively) and more variable when compared to values in healthy volunteers; the mean bupropion half-life was also longer (29 hours in patients with severe hepatic cirrhosis vs. 19 hours in healthy subjects). For the metabolite hydroxybupropion, the mean Cmax was approximately 69% lower. For the combined amino-alcohol isomers threohydrobupropion and erythrohydrobupropion, the mean Cmax was approximately 31% lower. The mean AUC increased by about 1½-fold for hydroxybupropion and about 2½-fold for threo/erythrohydrobupropion. The median Tmax was observed 19 hours later for hydroxybupropion and 31 hours later for threo/erythrohydrobupropion. The mean half-lives for hydroxybupropion and threo/erythrohydrobupropion were increased 5- and 2-fold, respectively, in patients with severe hepatic cirrhosis compared to healthy volunteers [see Dosage and Administration (2.4) and Use in Specific Populations (8.7) ].
Renal
There is limited information on the pharmacokinetics of bupropion in patients with renal impairment. An inter-study comparison between normal subjects and patients with end-stage renal failure demonstrated that the parent drug Cmax and AUC values were comparable in the 2 groups, whereas the hydroxybupropion and threohydrobupropion metabolites had a 2.3- and 2.8-fold increase, respectively, in AUC for patients with end-stage renal failure. The elimination of the major metabolites of bupropion may be reduced by impaired renal function. A second study, comparing normal subjects and patients with moderate-to-severe renal impairment (GFR 30.9 ± 10.8 mL/min) showed that exposure to a single 150 mg dose sustained-release bupropion was approximately 2-fold higher in patients with impaired renal function while levels of the hydroxybupropion and threo/erythrohydrobupropion (combined) metabolites were similar in the 2 groups. The elimination of bupropion and/or the major metabolites of bupropion may be reduced by impaired renal function [see Dosage and Administration (2.5) and Use in Specific Populations (8.6) ].
Left Ventricular Dysfunction
During a chronic dosing study with bupropion in 14 depressed patients with left ventricular dysfunction (history of CHF or an enlarged heart on x-ray), no apparent effect on the pharmacokinetics of bupropion or its metabolites was revealed, compared to healthy volunteers.
Age
The effects of age on the pharmacokinetics of bupropion and its metabolites have not been fully characterized, but an exploration of steady-state bupropion concentrations from several depression efficacy studies involving patients dosed in a range of 300 to 750 mg/day, on a 3 times daily schedule, revealed no relationship between age (18 to 83 years) and plasma concentration of bupropion. A single-dose pharmacokinetic study demonstrated that the disposition of bupropion and its metabolites in elderly subjects was similar to that of younger subjects. These data suggest there is no prominent effect of age on bupropion concentration; however, another pharmacokinetic study, single and multiple dose, has suggested that the elderly are at increased risk for accumulation of bupropion and its metabolites [see Use in Specific Populations (8.5) ].
Gender
A single-dose study involving 12 healthy male and 12 healthy female volunteers revealed no sex-related differences in the pharmacokinetic parameters of bupropion.
Smokers
The effects of cigarette smoking on the pharmacokinetics of bupropion hydrochloride were studied in 34 healthy male and female volunteers; 17 were chronic cigarette smokers and 17 were nonsmokers. Following oral administration of a single 150-mg dose of bupropion, there was no statistically significant difference in Cmax, half-life, Tmax, AUC, or clearance of bupropion or its active metabolites between smokers and nonsmokers.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Lifetime carcinogenicity studies were performed in rats and mice at doses up to 300 and 150 mg/kg/day bupropion hydrochloride, respectively. These doses are approximately 7 and 2 times the maximum recommended human dose (MRHD), respectively, on a mg/m2 basis. In the rat study there was an increase in nodular proliferative lesions of the liver at doses of 100 to 300 mg/kg/day of bupropion hydrochloride (approximately 2 to 7 times the MRHD on a mg/m2 basis); lower doses were not tested. The question of whether or not such lesions may be precursors of neoplasms of the liver is currently unresolved. Similar liver lesions were not seen in the mouse study, and no increase in malignant tumors of the liver and other organs was seen in either study.
Bupropion produced a positive response (2 to 3 times control mutation rate) in 2 of 5 strains in one Ames bacterial mutagenicity assay, but was negative in another. Bupropion produced an increase in chromosomal aberrations in 1 of 3 in vivo rat bone marrow cytogenetic studies.
A fertility study in rats at doses up to 300 mg/kg/day revealed no evidence of impaired fertility.
14 CLINICAL STUDIES
The efficacy of bupropion as a treatment for major depressive disorder was established with the immediate-release formulation of bupropion hydrochloride in two 4-week, placebo-controlled trials in adult inpatients and in one 6-week, placebo-controlled trial in adult outpatients. In the first study, patients were titrated in a bupropion hydrochloride dose range of 300 to 600 mg/day of the immediate-release formulation on a 3 times daily schedule; 78% of patients received maximum doses of 450 mg/day or less. This trial demonstrated the effectiveness of bupropion on the Hamilton Depression Rating Scale (HDRS) total score, the depressed mood item (item 1) from that scale, and the Clinical Global Impressions (CGI) severity score. A second study included 2 fixed doses of the immediate-release formulation of bupropion hydrochloride (300 and 450 mg/day) and placebo. This trial demonstrated the effectiveness of bupropion, but only at the 450 mg/day dose of the immediate-release formulation; the results were positive for the HDRS total score and the CGI severity score, but not for HDRS item 1. In the third study, outpatients received 300 mg/day of the immediate-release formulation of bupropion hydrochloride. This study demonstrated the effectiveness of bupropion on the HDRS total score, HDRS item 1, the Montgomery-Asberg Depression Rating Scale, the CGI severity score, and the CGI improvement score.
In a longer-term study, outpatients meeting DSM-IV criteria for major depressive disorder, recurrent type, who had responded during an 8-week open trial on bupropion hydrochloride (150 mg twice daily of the sustained-release formulation) were randomized to continuation of their same dose of bupropion or placebo, for up to 44 weeks of observation for relapse. Response during the open phase was defined as CGI Improvement score of 1 (very much improved) or 2 (much improved) for each of the final 3 weeks. Relapse during the double-blind phase was defined as the investigator’s judgment that drug treatment was needed for worsening depressive symptoms. Patients receiving continued bupropion treatment experienced significantly lower relapse rates over the subsequent 44 weeks compared to those receiving placebo.
Although there are no independent trials demonstrating the antidepressant effectiveness of bupropion extended release studies have demonstrated similar bioavailability of bupropion extended-release to both the immediate-release formulation and to the sustained-release formulation of bupropion under steady-state conditions, i.e., WELLBUTRIN XL® 300 mg once daily was shown to have bioavailability that was similar to that of 100 mg 3 times daily of the immediate-release formulation of bupropion and to that of 150 mg 2 times daily of the sustained-release formulation of bupropion, with regard to both peak plasma concentration and extent of absorption, for parent drug and metabolites. Further, it has been demonstrated that FORFIVO XL is bioequivalent to WELLBUTRIN XL®.
16 HOW SUPPLIED/STORAGE AND HANDLING
FORFIVO XL Extended-Release Tablets, 450 mg of bupropion hydrochloride, are white to off white, oblong shaped tablets printed with the “Forfivo” logo on one side supplied in bottles of 30 tablets
(NDC 49909-010-30). °
Store at 20 °C to 25 °C (68 °F to 77 °F) [See USP Controlled Room Temperature].
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Medication Guide).
17.1 Counseling Information
Prescribers or other health professionals should inform patients, their families, and their caregivers about the benefits and risks associated with treatment with FORFIVO XL and should counsel them in its appropriate use. A patient Medication Guide about “Antidepressant Medicines, Depression and Other Serious Mental Illnesses, and Suicidal Thoughts or Actions”, "Quitting Smoking, Quit-Smoking Medication, Changes in Thinking and Behavior, Depression, and Suicidal Thoughts or Actions” and “What Other Important Information Should I Know about FORFIVO XL” is available for FORFIVO XL. The prescriber or health professional should instruct patients, their families, and their caregivers to read the Medication Guide and should assist them in understanding its contents. Patients should be given the opportunity to discuss the contents of the Medication Guide and to obtain answers to any questions they may have. The complete text of the Medication Guide is reprinted at the end of this document. Patients should be advised of the following issues and asked to alert their prescriber if these occur while taking FORFIVO XL.
Clinical Worsening and Suicide Risk in Treating Psychiatric Disorders: Patients, their families, and their caregivers should be encouraged to be alert to the emergence of anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania, mania, other unusual changes in behavior, worsening of depression, and suicidal ideation, especially early during antidepressant treatment and when the dose is adjusted up or down. Families and caregivers of patients should be advised to observe for the emergence of such symptoms on a day-to-day basis, since changes may be abrupt. Such symptoms should be reported to the patient’s prescriber or health professional, especially if they are severe, abrupt in onset, or were not part of the patient’s presenting symptoms. Symptoms such as these may be associated with an increased risk for suicidal thinking and behavior and indicate a need for very close monitoring and possibly changes in the medication.
Neuropsychiatric Symptoms and Suicide Risk in Smoking Cessation Treatment: Although FORFIVO XL is not indicated for smoking cessation treatment, it contains the same active ingredient as ZYBAN which is approved for this use. Patients should be informed that quitting smoking, with or without ZYBAN, may be associated with nicotine withdrawal symptoms (including depression or agitation), or exacerbation of pre-existing psychiatric illness. Furthermore, some patients have experienced changes in mood (including depression and mania), psychosis, hallucinations, paranoia, delusions, homicidal ideation, aggression, anxiety, and panic, as well as suicidal ideation and completed suicide when attempting to quit smoking while taking ZYBAN.
Bupropion-Containing Products: Patients should be made aware that FORFIVO XL contains the same active ingredient (bupropion) found in ZYBAN, which is used as an aid to smoking cessation treatment, and that FORFIVO XL should not be used in combination with ZYBAN or any other medications that contain bupropion hydrochloride (such as WELLBUTRIN XL®, the extended-release formulation, WELLBUTRIN SR, the sustained-release formulation, WELLBUTRIN, the immediate-release formulation) or APLENZIN, Extended Release tablet.
Patients should be told that FORFIVO XL should be discontinued and not restarted if they experience a seizure while on treatment.
Patients should be told that any CNS-active drug like FORFIVO XL tablets may impair their ability to perform tasks requiring judgment or motor and cognitive skills. Consequently, until they are reasonably certain that FORFIVO XL tablets do not adversely affect their performance, they should refrain from driving an automobile or operating complex, hazardous machinery.
Patients should be told that the excessive use or abrupt discontinuation of alcohol or sedatives (including benzodiazepines) may alter the seizure threshold. Some patients have reported lower alcohol tolerance during treatment with bupropion. Patients should be advised that the consumption of alcohol should be minimized or avoided.
Patients should be advised to notify their physicians if they are taking or plan to take any prescription or over-the-counter drugs. Concern is warranted because FORFIVO XL tablets and other drugs may affect each other’s metabolism.
Patients should be advised to notify their physicians if they become pregnant or intend to become pregnant during therapy.
Patients should be advised to swallow FORFIVO XL tablets whole so that the release rate is not altered. Do not chew, divide, or crush tablets, as this may lead to an increased risk of adverse effects, including seizures.
The following are registered trademarks of their respective manufacturers: WELLBUTRIN® and WELLBUTRIN XL®/GlaxoSmithKline; ZYBAN® /GlaxoSmithKline; KALETRA® /Abbott Laboratories.
Manufactured by:
Pillar5 Pharma Inc.
Arnprior, Ontario, K7S 0C9
Canada
For:
Edgemont Pharmaceuticals, LLC
Austin, TX 78746
Revised January 2013
FFO_V2
MEDICATION GUIDE
FORFIVO XL
Fore fye' voe Eks el
(bupropion hydrochloride extended-release tablets)
Read this Medication Guide carefully before you start using FORFIVO XL and each time you get a refill. There may be new information. This information does not take the place of talking with your healthcare provider about your medical condition or your treatment. If you have any questions about FORFIVO XL, ask your healthcare provider or pharmacist.
IMPORTANT: Be sure to read the three sections of this Medication Guide. The first section is about the risk of suicidal thoughts and actions with antidepressant medicines; the second section is about the risk of changes in thinking and behavior, depression and suicidal thoughts or actions with medicines used to quit smoking; and the third section is entitled “What other important information should I know about FORFIVO XL?”
Antidepressant Medicines, Depression and Other Serious Mental Illnesses, and Suicidal Thoughts or Actions
This section of the Medication Guide is only about the risk of suicidal thoughts and actions with antidepressant medicines. Talk to your, healthcare provider or your family member’s healthcare provider about:
- all risks and benefits of treatment with antidepressant medicines
- all treatment choices for depression or other serious mental illness
What is the most important information I should know about antidepressant medicines, depression and other serious mental illnesses, and suicidal thoughts or actions?
- Antidepressant medicines may increase suicidal thoughts or actions in some children, teenagers, and young adults within the first few months of treatment.
- Depression and other serious mental illnesses are the most important causes of suicidal thoughts and actions. Some people may have a particularly high risk of having suicidal thoughts or actions. These include people who have (or have a family history of) bipolar illness (also called manic-depressive illness) or suicidal thoughts or actions.
-
How can I watch for and try to prevent suicidal thoughts and actions in myself or a family member?
- Pay close attention to any changes, especially sudden changes in mood, behaviors, thoughts, or feelings. This is very important when an antidepressant medicine is started or when the dose is changed.
- Call the healthcare provider right away to report new or sudden changes in mood, behavior, thoughts, or feelings.
- Keep all follow-up visits with the healthcare provider as scheduled. Call the healthcare provider between visits as needed, especially if you have concerns about symptoms.
Call a healthcare provider right away if you or your family member has any of the following symptoms, especially if they are new, worse, or worry you:
|
|
What else do I need to know about antidepressant medicines?
- Never stop an antidepressant medicine without first talking to a healthcare provider. Stopping an antidepressant medicine suddenly can cause other symptoms.
- Antidepressants are medicines used to treat depression and other illnesses. It is important to discuss all the risks of treating depression and also the risks of not treating it. Patients and their families or other caregivers should discuss all treatment choices with the healthcare provider, not just the use of antidepressants.
- Antidepressant medicines have other side effects. Talk to the healthcare provider about the side effects of the medicine prescribed for you or your family member.
- Antidepressant medicines can interact with other medicines. Know all of the medicines that you or your family member takes. Keep a list of all medicines to show the healthcare provider. Do not start new medicines without first checking with your healthcare provider.
- Not all antidepressant medicines prescribed for children are FDA approved for use in children. Talk to your child’s healthcare provider for more information.
It is not known if FORFIVO XL is safe and effective in children.
Quitting Smoking, Quit-Smoking Medications, Changes in Thinking and Behavior, Depression, and Suicidal Thoughts or Actions
This section of the Medication Guide is only about the risk of changes in thinking and behavior, depression and suicidal thoughts or actions with drugs used to quit smoking.
Although FORFIVO XL is not a treatment for quitting smoking, it contains the same active ingredient (bupropion hydrochloride) as ZYBAN® which is used to help patients quit smoking.
Some people have had changes in behavior, hostility, agitation, depression, suicidal thoughts or actions while taking bupropion to help them quit smoking. These symptoms can develop during treatment with bupropion or after stopping treatment with bupropion.
If you, your family member, or your caregiver notice agitation, hostility, depression or changes in thinking or behavior that are not typical for you, or you have any of the following symptoms, stop taking bupropion and call your healthcare provider right away:
- thoughts about suicide or dying
- attempts to commit suicide
- new or worse depression
- new or worse anxiety
- panic attacks
- feeling very agitated or restless
- acting aggressive, being angry, or violent
- acting on dangerous impulses
- an extreme increase in activity and talking (mania)
- abnormal thoughts or sensations
- seeing or hearing things that are not there (hallucinations)
- feeling people are against you (paranoia)
- feeling confused
- other unusual changes in behavior or mood
When you try to quit smoking, with or without bupropion, you may have symptoms that may be due to nicotine withdrawal, including urge to smoke, depressed mood, trouble sleeping, irritability, frustration, anger, feeling anxious, difficulty concentrating, restlessness, decreased heart rate, and increased appetite or weight gain.
Some people have even experienced suicidal thoughts when trying to quit smoking without medication. Sometimes quitting smoking can lead to worsening of mental health problems that you already have, such as depression.
Before taking bupropion, tell your healthcare provider if you have ever had depression or other mental illnesses. You should also tell your healthcare provider about any symptoms you had during other times you tried to quit smoking, with or without bupropion.
WHAT OTHER IMPORTANT INFORMATION SHOULD I KNOW ABOUT FORFIVO XL?
- Activation of mania/hypomania: Depression may be the early symptom of bipolar disorder. Using FORFIVO XL can trigger mania/hypomania if you have bipolar disorder. Tell your healthcare provider all of your symptoms, and if you or a family member has ever had depression, suicidal thoughts or actions, bipolar disorder or other mental health problems. Your healthcare provider may check you for bipolar disorder.
-
Seizures: There is a chance of having a seizure (convulsion, fit) with FORFIVO XL, especially in people:
- with certain medical problems.
- who take certain medicines.
For more information, see the sections “Who should not take FORFIVO XL?” and “What should I tell my healthcare provider before using FORFIVO XL?” Tell your healthcare provider about all of your medical conditions and all the medicines you take. Do not take any other medicines while you are using FORFIVO XL unless your healthcare provider has said it is okay to take them.
If you have a seizure while taking FORFIVO XL, stop taking the tablets and call your healthcare provider right away. Do not take FORFIVO XL again if you have a seizure.
- High blood pressure (hypertension): Some people get high blood pressure that can be severe, while taking FORFIVO XL. The chance of high blood pressure may be higher if you also use nicotine replacement therapy (such as a nicotine patch) to help you stop smoking.
- Severe allergic reactions: Some people have severe allergic reactions to FORFIVO XL. Stop taking FORFIVO XL and call your healthcare provider right away if you get a rash, itching, hives, fever, swollen lymph glands, painful sores in the mouth or around the eyes, swelling of the lips or tongue, chest pain, or have trouble breathing. These could be signs of a serious allergic reaction.
- Unusual thoughts or behaviors: Some patients have unusual thoughts or behaviors while taking FORFIVO XL, including delusions (believe you are someone else), hallucinations (seeing or hearing things that are not there), paranoia (feeling that people are against you), or feeling confused. If this happens to you, call your healthcare provider.
What is FORFIVO XL?
FORFIVO XL is a prescription medicine used to treat adults with a certain type of depression called major depressive disorder.
Who should not take FORFIVO XL?
Do not take FORFIVO XL if you:
- have or had a seizure disorder or epilepsy.
- are taking ZYBAN® (used to help people stop smoking) or any other medicines that contain bupropion, such as WELLBUTRIN ® Tablets, or WELLBUTRIN SR® Sustained-Release Tablets, or WELLBUTRIN XL® Extended-Release Tablets or APLENZIN EXTENDED-RELEASE TABLETS. Bupropion is the same active ingredient that is in FORFIVO XL.
- abruptly stop drinking, or use medicines called sedatives (these make you sleepy) or benzodiazepines and you stop using them all of a sudden.
- have taken within the last 14 days medicine for depression called a monoamine oxidase inhibitor (MAOI), such as NARDIL® (phenelzine sulfate), PARNATE® (tranylcypromine sulfate), or MARPLAN® (isocarboxazid).
- have or had an eating disorder such as anorexia nervosa or bulimia.
- are allergic to the active ingredient in FORFIVO XL, bupropion, or to any of the inactive ingredients. See the end of this leaflet for a complete list of ingredients in FORFIVO XL.
What should I tell my healthcare provider before using FORFIVO XL?
- Tell your healthcare provider if you have ever had depression, suicidal thoughts or actions, or other mental health problems. See "Antidepressant Medicines, Depression and Other Serious Mental Illnesses, and Suicidal Thoughts or Actions."
-
Tell your healthcare provider about your other medical conditions. Tell your healthcare provider if you:
- are pregnant or plan to become pregnant. It is not known if FORFIVO XL can harm your unborn baby.
- are breastfeeding. FORFIVO XL passes through your milk. It is not known if FORFIVO XL can harm your baby.
- have liver problems, especially cirrhosis of the liver.
- have kidney problems.
- have an eating disorder such as anorexia nervosa or bulimia.
- have had a head injury.
- have had a seizure (convulsion, fit).
- have a tumor in your nervous system (brain or spine).
- have had a heart attack, heart problems, or high blood pressure.
- are a diabetic taking insulin or other medicines to control your blood sugar.
- drink alcohol.
- abuse prescription medicines or street drugs.
- Tell your healthcare provider about all the medicines you take, including prescription and non-prescription medicines, vitamins and herbal supplements. Many medicines increase your chances of having seizures or other serious side effects if you take them while you are using FORFIVO XL.
How should I take FORFIVO XL?
- Take FORFIVO XL exactly as prescribed by your doctor.
- Do not chew, cut, or crush FORFIVO XL tablets. If you do, the medicine will be released into your body too quickly. If this happens, you may be more likely to get side effects including seizures. You must swallow the tablets whole. Tell your healthcare provider if you cannot swallow medicine tablets.
- You may take FORFIVO XL with or without food.
- If you take too much FORFIVO XL, or overdose, call your healthcare provider or go to the nearest hospital emergency room right away.
- Do not take any other medicines while using FORFIVO XL unless your healthcare provider has told you it is okay.
- If you are taking FORFIVO XL for the treatment of major depressive disorder, it may take several weeks for you to feel that FORFIVO XL is working. Once you feel better, it is important to keep taking FORFIVO XL exactly as directed by your doctor. Call your healthcare provider if you do not feel FORFIVO XL is working for you.
- Do not change your dose or stop taking FORFIVO XL without talking with your healthcare provider first.
What should I avoid while taking FORFIVO XL?
- Do not drink alcohol while taking FORFIVO XL.
- Do not drive a car or use heavy machinery until you know how FORFIVO XL affects you. FORFIVO XL can impair your ability to perform these tasks.
What are possible side effects of FORFIVO XL?
FORFIVO XL can cause serious side effects, including:
- See “What is the most important information I should know about FORFIVO XL?”
The most common side effects of FORFIVO XL include:
|
|
If you have nausea, take your medicine with food. If you have trouble sleeping, do not take your medicine too close to bedtime.
Tell your healthcare provider right away about any side effects that bother you.
These are not all the side effects of FORFIVO XL. For a complete list, ask your healthcare provider or pharmacist.
Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store FORFIVO XL?
- Store FORFIVO XL between 68 °F to 77 °F (20 °C to 25 °C).
Keep FORFIVO XL and all medicines out of the reach of children.
General Information about FORFIVO XL.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use FORFIVO XL for a condition for which it was not prescribed. Do not give FORFIVO XL to other people, even if they have the same symptoms you have. It may harm them.
If you take a urine drug screening test, FORFIVO XL may make the test result positive for amphetamines. If you tell the person giving you the drug screening test that you are taking FORFIVO XL, they can do a more specific drug screening test that should not have this problem.
This Medication Guide summarizes important information about FORFIVO XL. For more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about FORFIVO XL that is written for health professionals.
For more information, call 1-888-594-4332.
What are the ingredients in FORFIVO XL?
Active ingredient: bupropion hydrochloride.
Inactive ingredients: hydroxypropyl cellulose, hydrochloric acid, polyvinyl pyrrolidone and polyvinyl acetate blend, polyethylene oxide, stearic acid, colloidal silicon dioxide, magnesium stearate, hydroxypropylmethyl cellulose, triacetin, talc, methacrylic acid copolymer, polyethylene glycol 8000, titanium dioxide and carboxymethyl cellulose sodium. The tablets are printed with edible black ink.
The following are registered trademarks of their respective manufacturers: WELLBUTRIN® and WELLBUTRIN XL®/GlaxoSmithKline; ZYBAN® /GlaxoSmithKline; PARNATE® / GlaxoSmithKline; NARDIL®/Warner Lambert Company; MARPLAN®/ Validus Pharmaceuticals LLC; KALETRA® /Abbott Laboratories.
Rx only
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Manufactured by:
Pillar5 Pharma Inc.
Arnprior, Ontario, K7S 0C9
Canada
For:
Edgemont Pharmaceuticals, LLC
Austin, TX 78746
Revised January 2013
FFO_V2
PRINCIPAL DISPLAY PANEL

NDC 49909-010-30 Rx only
Forfivo XL™
(bupropion hydrochloride
extended-release tablets)
450 mg
30 Tablets
EDGEMONT
PHARMACEUTICALS
Each Tablet Contains:
Bupropion Hydrochloride, 450 mg
Extended-Release Tablet
USUAL DOSAGE:
Take 1 tablet daily or as directed by
physician. Consult package insert
for full prescribing information.
Storage: Store at 20°C to 25°C (68°F to 77°F)
[See USP Controlled Room Temperature].
WARNING: Do not use with other medicines
that contain bupropion hydrochloride or
bupropion hydrobromide.
KEEP THIS AND ALL MEDICATIONS
OUT OF REACH OF CHILDREN
Manufactured by:
Pillar5 Pharma Inc.
365 Madawaska Blvd.
Arnprior, Ontario K7S 0C9 CANADA
For:
Edgemont Pharmaceuticals, LLC.
Austin, TX 78746
Medication Guide to be dispensed with
each prescription.
DO NOT USE if safety seal under cap is
broken or missing.
70119-05-2 Rev. 02
Forfivobupropion hydrochloride TABLET, FILM COATED, EXTENDED RELEASE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||