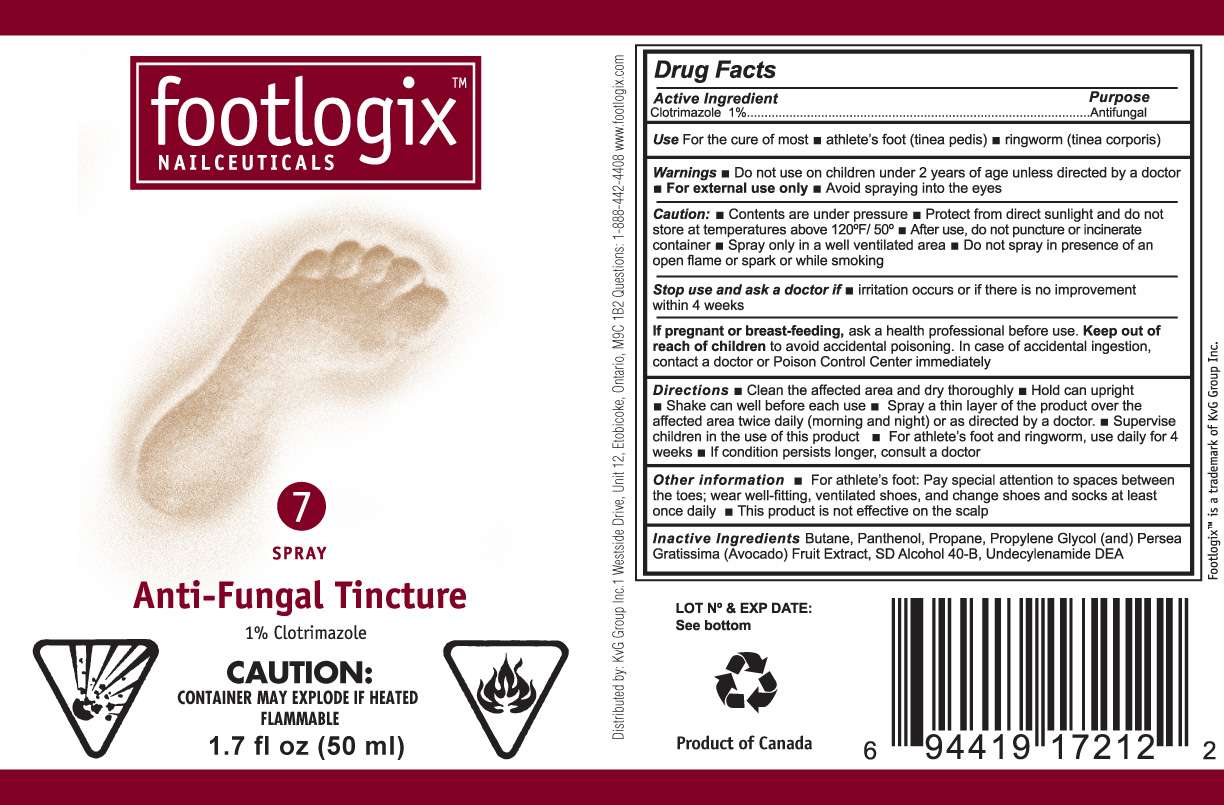
footlogix
KVG Group Inc
Drug Facts
FULL PRESCRIBING INFORMATION
Active ingredient
Active Ingredient
Purpose
Purpose
Uses
Use
- For the cure of most athlete’s foot (tinea pedis)
- ringworm (tinea corporis)
Warnings
-
For external use only
-
Do
not use on children under 2 years of age unless directed by a doctor
-
For
external use only
-
Avoid spraying into the eyes
Caution
:
- Contents are under pressure
- Protect from direct sunlight and do not store at temperatures above 120F/50C
- After use, do not puncture or incinerate container
- Spray only in a well ventilated area
- Do not spray in presence of open flame or spark or while smoking
Stop use and ask a doctor if
-
irritation occurs or if there is no improvement within 4 weeks
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children to avoid accidental poisoning. In case of accidental ingestion, contact a doctor or Poison Control Center immediately.
Directions
-
Clean the affected
area and dry thoroughly
-
Hold
can upright
-
Shake
can well before each use
-
Spray
a thin layer of the product over the affected area twice daily (morning and
night) or as directed by a doctor.
-
Supervise children in the use of
this product
-
For athlete’s foot and ringworm, use
daily for 4 weeks
-
If condition persists longer,
consult a doctor
Other information
-
For athlete’s foot Pay special
attention to spaces between the toes; wear well-fitting, ventilated shoes, and
change shoes and socks at least once daily.
-
This product is not effective on the scalp
I
n
active Ingredients
footlogix
Clotrimazole AEROSOL, SPRAY
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:42479-212 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
Clotrimazole CLOTRIMAZOLE |
|
1 mL
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:42479-212-17 |
50 in 1 CAN |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part333 |
2010-09-01 |
|
|
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!
