First Aid Burn
First Aid Burn Cream
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredients
- Purpose
- First Aid Burn Uses
- Warnings
- Directions
- First Aid Burn Other information
- Inactive ingredients
- Questions?
- Principal Display Panel
FULL PRESCRIBING INFORMATION
Active ingredients
Benzalkonium chloride 0.13%
Lidocaine HCl 0.5%
Purpose
First Aid antiseptic
External analgesic
First Aid Burn Uses
first aid to help prevent infection and for the temporary relief of pain and itching associated with
- minor cuts
- scrapes
- burns
Warnings
For external use only
Do not use
- in the eyes or apply over large areas of the body
- longer than 1 week unless directed by a doctor
- in large quantities, particularly over raw surfaces or blistered areas
Ask a doctor before use if you have
deep or puncture wounds, animal bites, or serious burns
When using this product
avoid contact with eyes
Stop use and ask a doctor if
- condition worsens
- symptoms persist for more than 7 days
- condition clears up and occurs again within a few days
Keep out of the reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- adults and children 2 years of age and older:
- clean the affected area
- apply a small amount of this product on the area 1 to 3 times daily
- may be covered with a sterile bandage
- children under 2 years of age: consult a doctor
First Aid Burn Other information
- store at room temperature (do not freeze)
- tamper evident sealed packets
- do not use if packet is opened or torn
- you may report a serious reaction to this product to 800-275-3433
Inactive ingredients
water, mineral oil, stearic acid, glyceryl stearate se, glycerin, Peg-100, cetyl alcohol, propylene glycol, diazolidinyl urea, methylparaben, propylparaben, aloe barbadensis leaf juice, triethanolamine, disodium EDTA
Questions?
800-275-3433 info@waterjel.com www.waterjel.com
Principal Display Panel
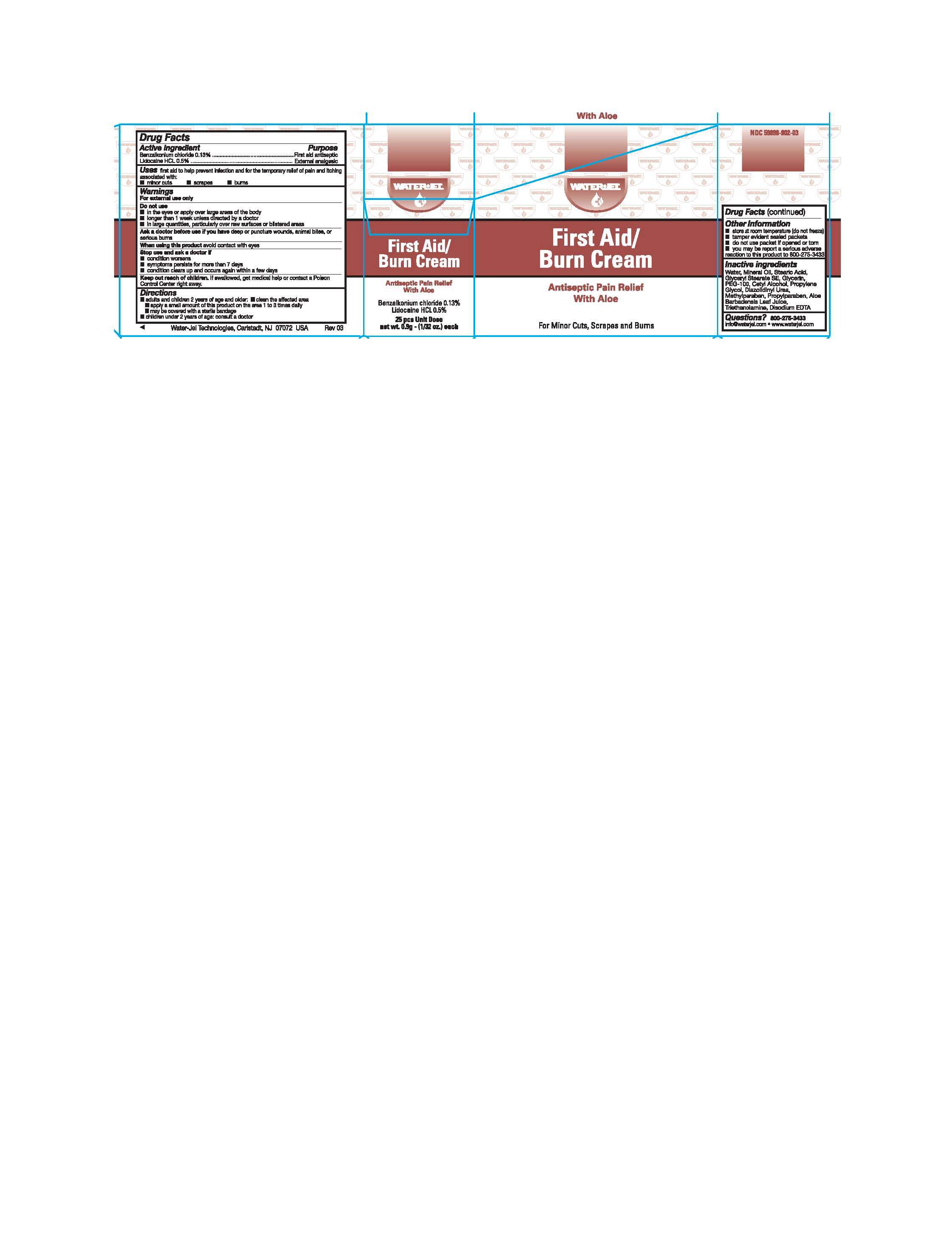
First Aid BurnBenzalkonium Chloride, Lidocaine Hydrochloride CREAM
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||