FIRMING DAY
FULL PRESCRIBING INFORMATION
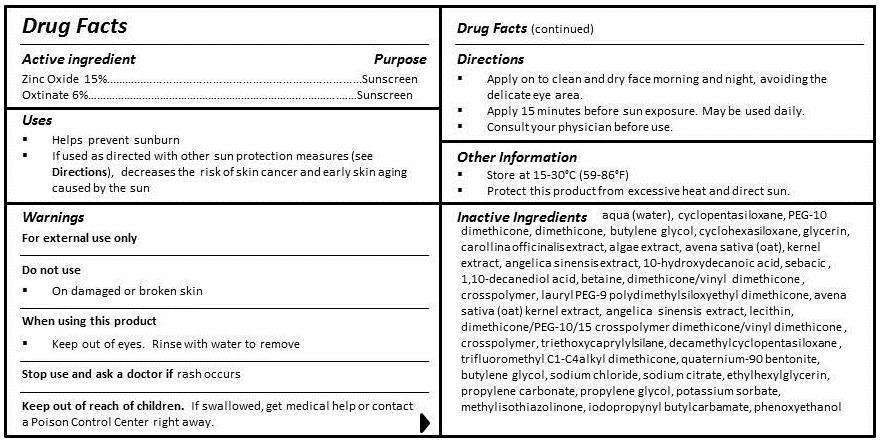
Active ingredient
ACTIVE INGREDIENT
ZINC OXIDE 15%
OCTINOXATE 6%
Purpose
PURPOSE
SUNSCREEN
Uses
USES
- Helps prevent sunburn
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
WARNINGS
FOR EXTERNAL USE ONLY
DO NOT USE
-
On damaged or broken skin
WHEN USING THIS PRODUCT
- Keep out of eyes. Rinse with water to remove.
STOP USE AND ASK A DOCTOR IF RASH OCCURS.
KEEP OUT OF REACH OF CHILDREN. IF SWALLOWED, GET MEDICAL HELP OR CONTACT A POISON CONTROL CENTER RIGHT AWAY.
DIRECTIONS
- Apply on to clean and dry face morning and night, avoiding the delicate eye area.
- Apply 15 minutes before sun exposure. May be used daily.
- Consult your physician before use.
Other Information
- STORE AT 15-30°C (59-86°F)
- PROTECT THIS PRODUCT FROM EXCESSIVE HEAT AND DIRECT SUN.
INGREDIENTS
aqua (water), cyclopentasiloxane, PEG-10 dimethicone, dimethicone, butylene glycol, cyclohexasiloxane, glycerin, carollina officinalis extract, algae extract, avena sativa (oat) kernel extract, angelica sinensis extract, 10-hydroxydecanoic acid, sebacic acid, 1,10-decanediol acid, betaine, dimethicone/vinyl dimethicone crosspolymer, lauryl PEG-9 polydimethylsiloxyethyl dimethicone, lecithin, dimethicone/PEG-10/15 crosspolymer, triethoxycaprylylsilane, decamethylcyclopentasiloxane, trifluoromethyl C1-C4 alkyl dimethicone, quaternium-90, bentonite, butylene glycol, sodium chloride, sodium citrate, ethylhexylglycerin, propylene carbonate, propylene glycol, potassium sorbate, methylisothiazolinone, iodopropynyl butylcarbamate, phenoxyethanol